Search Your Topic
Acid- base balance- Lecture 2 (role of blood buffers)
Buffers
Buffers are weak acids or bases that are able to minimize changes in pH by taking up or releasing H+. Phosphate is an example of an effective buffer, as in the following reaction:
HPO4 2- + (H+) «H2 PO4 –
Upon addition of an H+ to extracellular fluids, the monohydrogen phosphate binds H+ to form dihydrogen phosphate, minimizing the change in pH. Similarly, when [H+] is decreased, the reaction is shifted to the left. Thus, buffers work as the first line of defense to blunt the changes in pH that would otherwise result from the constant daily addition of acids and bases to body fluids. With the constant pouring in H+, the concentration of the monohydrogen phosphate will ultimately diminish and the pH will start falling.
The major Buffer system of the body
(1) HCO3 –/H2 CO3 buffering system HCO3-/CO2 (bicarbonate/carbon dioxide)
(2) HPO42―/H2PO4― (phosphate),
(3) Organic Phosphate Esters, and
(4) Proteins.
Proteins with side chains that contain more carboxyl terminal groups than amino-terminal groups promote an acidic environment. Proteins with side chains that contain more amino-terminal groups than carboxyl terminal groups promote an alkaline environment. Protein with side chains containing equal numbers of amino and carboxyl side groups are neutral, not affecting the pH.
Details of Buffers
(1) HCO3 –/H2 CO3 buffering system
In the ECF bicarbonate buffer is the most important buffer. Its function is illustrated by the following reactions:
H2 O + CO2 «H2 CO3 «H+ + HCO3 –
When an acid load (H+) is added to the body fluids, it results in the consumption of HCO3 – by the added H+. Carbonic acid thus formed, in turn, forms water and CO2. The CO2 concentration is maintained within a narrow range via the respiratory drive, which eliminates accumulating CO2. The kidneys regenerate the HCO3 – consumed during this reaction.
Put simply, whereas simple buffers rapidly become ineffective as the association of the hydrogen ion and the weak anion of the weak acid reaches equilibrium, the bicarbonate system keeps working because the carbonic acid is removed as CO2. The limit to the effectiveness of the bicarbonate system is the initial concentration of bicarbonate. The acid-base status of the patient is assessed by the bicarbonate concentration in the plasma. The association of hydrogen ion with bicarbonate occurs rapidly but the dissociation of H2CO3 to CO2 and H2O is slow. This process is accelerated by the enzyme Carbonic anhydrase, which is present in the erythrocytes and in the kidney whenever this reaction is needed. Buffering at the expense of bicarbonate effectively removes hydrogen ions from ECF. CO2 is removed from the lungs and water assimilates in the ECF without producing any change in p H. The ECF contains a large amount of bicarbonate to the extent of 24 mmol/L, when the H+ concentration increases the bicarbonate concentration comes down since it is used up during the process of buffering.
This reaction continues to move to the left as long as CO2 is constantly eliminated or until HCO3 – is significantly depleted, making less HCO3 – available to bind H+. Since HCO3 – and PaCO2 can be managed independently (kidneys and lungs, respectively) that makes this a very effective buffering system. One of the major factors that make this system very effective is the ability to control PaCO2 by changes in ventilation. As can be noted from this reaction, increased carbon dioxide (CO2) concentration drives the reaction to the right, whereas a decrease in CO2 concentration drives it to the left.
Assessing acid-base status
An indication of the acid-base status of the patient can be determined by measuring the components of the bicarbonate system.
The Henderson-Hassel Balch equation describes the relationship between blood pH and the components of the H2 CO3 buffering system.
pH = 6.1 + log (HCO–3/ H2 CO3)
Bicarbonate (HCO3–) is in equilibrium with the metabolic components.
- Bicarbonate production in the kidney
- Acid production from endogenous or exogenous sources
Carbonic acid (H2 CO3) is in equilibrium with the respiratory component, as shown by the below equation:
H2 CO3 = PCO2 (mm Hg) X 0.03
Note that changes in pH or [H+] are a result of relative changes in the ratio of PaCO2 to [HCO3 –] rather than to an absolute change in either one. In other words, if both PaCO2 and [HCO3 –] change in the same direction, the ratio stays the same and the pH or [H+] remains relatively stable. To diminish the alteration in pH that occurs when either HCO3 – or PaCO2 changes, the body, within certain limits, changes the other variable in the same direction.
2) Phosphate buffer system (Na2HPO4/NaH2PO4)
The phosphate buffer system is directly linked up with the kidney.
Upon addition of acid, the H+ is neutralized by the Na2 HPO4 component forming NaH2PO4 that is eliminated through the kidney without any change in pH.
Na2 HPO4 + HCl–>> NaH2PO4 + NaCl
Similarly, upon addition of OH–, the acid component reacts to form, Na2 HPO4 that can be eliminated as well through the kidney without any change in pH
NaH2PO4 + Na OH—>> Na2 HPO4 + H2O
In other words, a Phosphate buffer system works in conjunction with the kidney.
Chemically it is a very good buffer, as pKa is close to Physiological pH, but physiologically due to its less concentration (1.0 mmol/L as compared to bicarbonate 26-28 mmol/L), it is less efficient.
3) Role of Haemoglobin as a buffer
The buffering capacity of Hb is due to the presence of the “Imidazole” nitrogen group of Histidine. Oxygenated Hb is a stronger acid than deoxygenated Hb. The acidity of the medium favors the delivery of oxygen to the tissues. The alkalinity of the medium favors the oxygenation of Hb. The sequence of events that occur in lungs and tissues is as follows;
- In the lungs (figure-1)
The formation of oxyhemoglobin from deoxyhemoglobin must release H+, which will react with HCO3– to form H2CO3. Due to the low CO2 tension in the lungs H2CO3, dissociates to form CO2 and H2O.CO2 is then eliminated in the expired air.
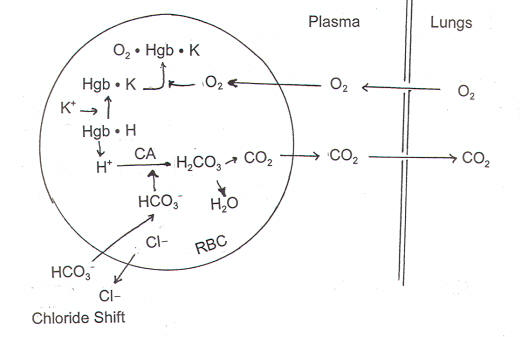
Figure-1- showing the role of Hb as a buffer in the lungs
- In the tissues (figure-2)
Oxy Hb dissociates to give O2 to the tissues and the deoxy Hb (Reduced Hb) is formed. At the same time, CO2 produced as a result of metabolism is hydrated to form H2CO3, which ionizes to form H+ and HCO3-. Deoxy Hb acts as anion and accepts H+ to form acid reduced Hb.
4) Protein buffer system
The buffering capacity of plasma proteins is much less than Hb. In acidic medium, protein acts a base and NH2 group takes up H+ forming NH3+, protein becomes positively charged.
The reverse occurs in the alkaline medium. Acidic COOH to give H+ that neutralizes the OH– forming H2O. Overall protein becomes negatively charged in the alkaline medium

Figure-2- showing the role of Hb as a buffer at the tissue level.
