Search Your Topic
Reduced state of the respiratory chain carriers (Inhibition of electron transport chain)
As electrons are received and passed down the transport chain, the electron carriers are first reduced with the acceptance of the electron and then oxidized with loss of the electron. A patient poisoned by which of the following compounds has the most highly reduced state of most of the respiratory chain carriers?
A. Antimycin A
B. Rotenone
C. Carbon monoxide
D. Puromycin
E. Chloramphenicol
The right answer is (c) – Carbon monoxide.
Basic concept
The electron transport chain contains three proton pumps linked by two mobile electron carriers. At each of these three sites (NADH-Q reductase, cytochrome c reductase and cytochrome oxidase); the transfer of electrons down the chain powers the pumping of the protons across the inner mitochondrial membrane (figure).As electrons pass through complexes I, III, and IV, protons are pumped across the inner membrane from the matrix to the intermembrane space. This sets up an electrochemical gradient consisting of a proton gradient (chemical), and membrane potential (because the proton carries a positive charge). The electrochemical gradient represents a form of stored energy derived from the oxidation of NADH (or succinate). A physically distinct complex (ATP synthase or Complex V) at a separate location in the inner membrane can exploit the electrochemical gradient to carry out the endergonic ATP synthesis (by oxidative phosphorylation).
Inhibition of electron transport chain
The blockage of electron transfers by specific point inhibitors leads to a buildup of highly reduced carriers behind the block because of the inability to transfer electrons across the block.
Antimycin A blocks the complex III, Rotenone blocks complex I and Carbon monoxide (As well as cyanide, azide, hydrogen sulfide) block complex IV. Therefore a carbon monoxide inhibition leads to a highly reduced state of all the carriers of the chain. Puromycin and Chloramphenicol are inhibitors of protein synthesis and have no direct effect upon the electron transport chain.
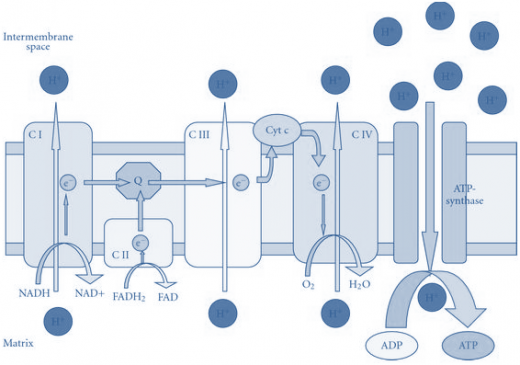
Figure-1- Flow of electrons in the electron transport chain and the oxidative phosphorylation
