Library
Disaccharides and Oligosaccharides
- November 9, 2019
- Posted by: Namrata Chhabra
- Category: Library Chemistry of Carbohydrates Learning resources Short-answer questions
Q.1- Give a brief account of the disaccharides of biological importance.
Answer- The disaccharides are sugars composed of two monosaccharide residues linked by a glycoside bond. The glycosidic bonds are readily hydrolyzed by acids but resist cleavage by alkaline hydrolysis. The glycosidic linkage is formed by loss of one molecule of water as shown in the reaction below The physiologically important disaccharides are as follows
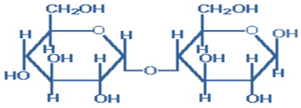
1) Maltose- It is also called ‘Malt sugar’ and is composed of 2 glucose monomers in an α-(1,4) glycosidic bond.
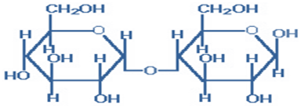
Figure-1- showing the structure of Maltose
It is produced by the partial hydrolysis of starch (either salivary amylase or pancreatic amylase) and is used as a nutrient (malt extract; Hordeum vulgare); as a sweetener and as a fermentative reagent. It is hydrolyzed to glucose by maltase.
Since it has a free active group hence- it is reducing in nature, can exist in α and
β-anomeric forms and can exhibit mutarotation.
2) Lactose- is also called ‘Milk Sugar’ and is composed of galactose joined to glucose by a β-1,4-glycosidic linkage.
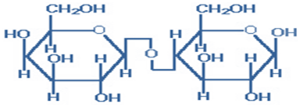
Figure-2- showing the structure of Lactose
Lactose is the only carbohydrate of milk (7gm% and 4gm% in human& bovine milk respectively). It is synthesized by mammary glands during lactation and is the best food for infants [Least sweet-laxative-non fermentable). It has a free active group, shows reducing properties and exhibits mutarotation.
Milk contains the α and β-anomers in a 2:3 ratio. β-lactose is sweeter and more soluble than ordinary α – lactose. It is used in infant formulations, medium for penicillin production and as a diluent in pharmaceuticals. Lactose is hydrolyzed to its component monosaccharides by lactase in human beings and by β -galactosidase in bacteria. Lack of lactase (alactasia) leads to lactose intolerance—diarrhea and flatulence; Lactose may be excreted in the urine in pregnancy
3) Sucrose- Sucrose (common table sugar) is obtained commercially from cane or beet. The anomeric carbon atoms of a glucose unit and a fructose unit are joined in this disaccharide; the configuration of this glycosidic linkage is α-for glucose and β-for fructose. Sucrose can be cleaved into its component monosaccharides by the enzyme sucrase. Sucrase, lactase, and maltase are located on the outer surfaces of epithelial cells lining the small intestine. Rare genetic lack of sucrase leads to sucrose intolerance— diarrhea and flatulence.

Figure-3- showing the structure of Sucrose.
Sucrose has no free reactive group because the anomeric carbons of both monosaccharides units are involved in the glycosidic bond. So, sucrose neither shows reducing nor mutarotation characters. Sucrose is called invert sugar because the optical activity of sucrose ( dextrorotatory) is inverted after hydrolysis [by an acid or an enzyme (invertase or sucrase)] into an equimolar mixture of its two components glucose (+52.5) and fructose (-92.5) and the optical activity of the mixture becomes levorotatory.
4) Lactulose- is composed of β-galactose and β fructose in a β -(1,4) glycosidic bond. It is a semi-synthetic disaccharide (not naturally occurring) and is not absorbed in the GI tract. It is used either as a laxative or in the management of portal-systemic encephalopathy. It is metabolized in distal ileum and colon by bacteria to lactic acid, formic acid, and acetic acid. In chronic liver diseases, the level of ammonia in the blood is increased, so, the oral Lactulose by microfloral conversion in the colon to organic acid will relieve the high ammonia. On the other hand, the osmotic activity of the disaccharide will cause diarrhea which will remove toxic products.
5) Isomaltose- is composed of two glucose units linked by α 1,6 glycosidic linkage. It is produced by enzymatic hydrolysis of starch (at the branch point in Amylopectin).
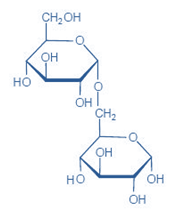
Figure-4- showing the structure of Isomaltose, 2 glucose units are linked together by α 1,6 glycosidic linkage
Q.2– Enlist the Oligosaccharides of biological importance
Answer- Oligosaccharides are condensation products of three to ten monosaccharides. Most are not digested by human enzymes. Some of the Oligosaccharides of biological importance are as follows-
•Trisaccharide: Raffinose (glucose, galactose, and fructose)
•Tetrasaccharide: Stachyose (2 galactose, glucose, and fructose)
•Pentasaccharide: Verbascose (3 galactose, glucose, and fructose)
•Hexasaccharide: Ajugose (4 galactose, glucose, and fructose)
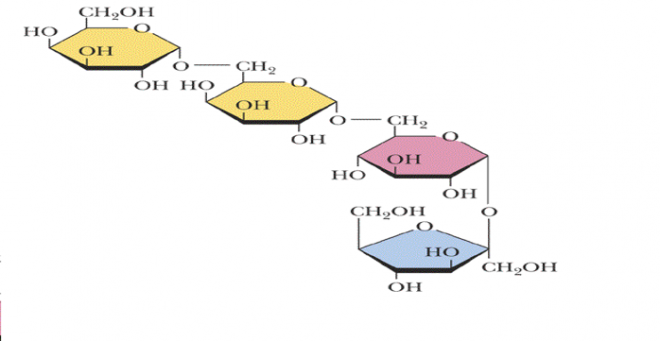

Figure-5- showing the structure of Stachyose (2 galactose, glucose, and fructose).
Stachyose is a constituent of many plants and is used to prevent constipation. Melezitose- a constituent of honey also contains Glucose, fructose, and some volatile oils. Cycloheptamylose- a breakdown product of starch is used in chromatographic procedures
.
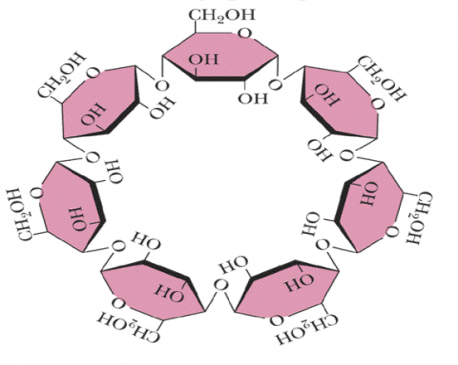

Figure –6-showing the structure of Cycloheptamylose
Oligosaccharides occur widely as components of antibiotics derived from various sources- e.g.- Bleomycin A2 (An antitumor agent) and Streptomycin used as broad-spectrum antibiotics are oligosaccharides.
Author:Namrata Chhabra
Leave a Reply Cancel reply
You must be logged in to post a comment.
