Library
Electron Transport Chain and Oxidative Phosphorylation- Multiple Choice Questions With Explanations-Set-2
- October 28, 2024
- Posted by: Namrata Chhabra
- Category: Bioenergetics Energy metabolism Learning resources Library Multiple-choice questions Multiple-choice questions Multiple-Choice questions Practice questions Practice questions Question Bank USMLE Content USMLE Style questions USMLE styled question bank
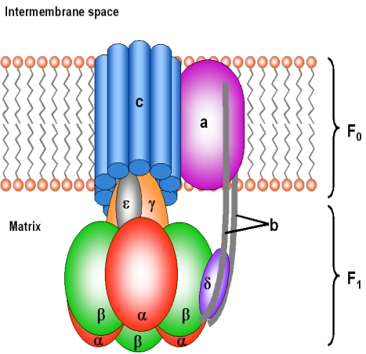
11. A 48-year-old patient is brought to the emergency room after exposure to toxic fumes suspected to contain cyanide. He presents with shortness of breath, confusion, and metabolic acidosis. The medical team suspects cyanide toxicity, which disrupts the electron transport chain (ETC). Which of the following statements concerning the components of the electron transport chain is true?
A. All of the components are embedded in the inner mitochondrial membrane
B. Cyanide does not inhibit proton pumping but inhibits ETC
C. Oxygen directly oxidizes cytochrome c
D. Succinate dehydrogenase directly reduces cytochrome c
Correct Option: B. Cyanide does not inhibit proton pumping but inhibits ETC. Cyanide inhibits Complex IV (cytochrome c oxidase) by preventing the transfer of electrons to oxygen, blocking the ETC. Although cyanide shuts down the electron flow, it does not directly affect the proton pumps. As a result, ATP production is halted, and cells shift to anaerobic metabolism, leading to metabolic acidosis, as seen in cyanide poisoning cases.
Incorrect Options:
A. All of the components are embedded in the inner mitochondrial membrane. Incorrect: While most components of the ETC are embedded in the inner mitochondrial membrane, cytochrome c is an exception. It is a mobile carrier that shuttles electrons along the outer surface of the inner membrane.
C. Oxygen directly oxidizes cytochrome c. Incorrect: Oxygen accepts electrons from Complex IV (cytochrome c oxidase), not from cytochrome c directly. Cytochrome c passes its electrons to Complex IV, where they are ultimately transferred to oxygen.
D. Succinate dehydrogenase directly reduces cytochrome c. Incorrect: Succinate dehydrogenase (Complex II) transfers electrons to ubiquinone (Coenzyme Q), not to cytochrome c. Cytochrome c receives electrons from Complex III (cytochrome bc1 complex).
12. A 35-year-old researcher accidentally exposes himself to oligomycin while studying mitochondrial function. Soon after, he experiences fatigue and muscle weakness due to impaired ATP production. The toxicology team explains that oligomycin disrupts mitochondrial ATP synthesis by blocking a key step in the electron transport chain. Which of the following statements best describes the mechanism of action of oligomycin?
A. It inhibits NADH dehydrogenase
B. It inhibits ATP/ADP transporter
C. It is an inhibitor of cytochrome oxidase
D. It blocks the flow of protons through the ATP synthase complex
E. It is an uncoupler of oxidative phosphorylation
Correct Option: D. It blocks the flow of protons through the ATP synthase complex. Oligomycin binds to the F₀ subunit of ATP synthase, preventing protons from flowing back into the mitochondrial matrix. This inhibition stops ATP synthesis since the proton gradient cannot drive the phosphorylation of ADP to ATP. As a result, cells experience an energy deficit, leading to fatigue and muscle weakness, as seen in the scenario.
Incorrect Options:
A. It inhibits NADH dehydrogenase. Incorrect: NADH dehydrogenase is part of Complex I in the electron transport chain. This step is inhibited by rotenone, not oligomycin.
B. It inhibits ATP/ADP transporter. Incorrect: The ATP/ADP transporter, also known as adenine nucleotide translocase (ANT), is inhibited by atractyloside, not oligomycin.
C. It is an inhibitor of cytochrome oxidase. Incorrect: Cytochrome oxidase (Complex IV) is inhibited by cyanide and hydrogen sulfide (H₂S), not oligomycin.
E. It is an uncoupler of oxidative phosphorylation. Incorrect: Uncouplers like 2,4-dinitrophenol (DNP) allow protons to bypass ATP synthase, generating heat but preventing ATP synthesis. Oligomycin, by contrast, blocks proton flow through ATP synthase, preventing ATP generation directly.
13. A 12-year-old boy with a history of developmental delays and seizures is brought to the emergency department with weakness and confusion. He is diagnosed with MELAS (Mitochondrial Encephalopathy, Lactic Acidosis, and Stroke-like episodes), a mitochondrial disorder affecting ATP production. Given the impaired function of mitochondria, the patient’s cells rely primarily on glycolysis for energy. What would be the net ATP yield from one molecule of glucose in this condition?
A. 0
B. 1
C. 2
D. 4
E. 8
Correct Option: C. 2. In MELAS and other mitochondrial disorders, oxidative phosphorylation in the mitochondria is impaired. As a result, the cells cannot utilize the electron transport chain or the citric acid cycle effectively. Therefore, the only energy source becomes glycolysis, which occurs in the cytoplasm and yields a net gain of 2 ATP per molecule of glucose.
Incorrect Options:
A. 0. Incorrect: Even without functional mitochondria, glycolysis still produces ATP, albeit much less efficiently than aerobic respiration. Hence, the net ATP yield is not zero.
B. 1. Incorrect: Glycolysis always produces a net of 2 ATP per glucose molecule, not 1.
D. 4. Incorrect: While glycolysis generates 4 ATP in total, the net yield is only 2 ATP because 2 ATP are consumed during the early steps of the pathway.
E. 8. Incorrect: This number is higher than what glycolysis alone can produce. Without functional mitochondria, ATP from oxidative phosphorylation (which would increase the yield to ~30-32 ATP) is unavailable.
14. A 6-year-old boy is evaluated by an ophthalmologist for difficulty seeing across all visual fields and slow eye movements. The examination reveals pigmentary retinopathy and ophthalmoplegia. Suspecting a mitochondrial disorder linked to a mutation in Complex II of the electron transport chain (ETC), the physician explains that the defect would impair electron transport from specific metabolic substrates. The electron transport from which of the following substances would be impaired?
A. Alpha-ketoglutarate
B. Isocitrate
C. Malate
D. Pyruvate
E. Succinate
Correct Option: E. Succinate: Complex II of the electron transport chain is also known as succinate dehydrogenase. It catalyzes the oxidation of succinate to fumarate, transferring electrons to ubiquinone (CoQ) in the process. A mutation in Complex II impairs this electron transfer, disrupting the flow of electrons into the ETC and ultimately impairing ATP production.
Incorrect Options:
A. Alpha-ketoglutarate: Alpha-ketoglutarate enters the citric acid cycle and is converted to succinyl-CoA, but its electrons are passed to NADH, which delivers electrons to Complex I, not Complex II.
B. Isocitrate: Isocitrate is part of the citric acid cycle, where it is converted into alpha-ketoglutarate. The electrons generated in this step are transferred via NADH to Complex I, not Complex II.
C. Malate: Malate is converted into oxaloacetate in the citric acid cycle, producing NADH, which delivers electrons to Complex I, not Complex II.
D. Pyruvate: Pyruvate is converted to acetyl-CoA by the pyruvate dehydrogenase complex, contributing to the citric acid cycle and generating NADH, which also transfers electrons to Complex I, not Complex II.
15. A 63-year-old man with a strong family history of Parkinson’s disease begins experiencing “pill-rolling tremors,” a hallmark sign of the condition. During a visit to his neurologist, he is informed about the potential benefits of coenzyme Q (ubiquinone) in slowing the progression of Parkinson’s disease. The neurologist explains that coenzyme Q is a critical component of the electron transport chain (ETC). Which of the following best describes the role of coenzyme Q in the ETC?
A. Contains heme
B. Receives electrons directly from Complex IV
C. Receives electrons directly from FMN
D. Receives electrons directly from NADH
E. Transports ATP to the cytoplasm
Correct Option: C. Receives electrons directly from FMN. Coenzyme Q (ubiquinone) acts as a mobile electron carrier within the electron transport chain. It receives electrons from FMN (flavin mononucleotide) in Complex I and also from Complex II (succinate dehydrogenase). Once reduced to ubiquinol, it transports electrons to Complex III (cytochrome bc1 complex).
Incorrect Options:
A. Contains heme. Incorrect: Coenzyme Q does not contain heme. Heme groups are found in cytochromes, such as those in Complex III and IV, not in coenzyme Q.
B. Receives electrons directly from Complex IV. Incorrect: Complex IV does not donate electrons to coenzyme Q. Instead, Complex IV transfers electrons to molecular oxygen, the final electron acceptor in the ETC.
D. Receives electrons directly from NADH. Incorrect: While NADH is a primary donor of electrons in the ETC, these electrons first pass through FMN in Complex I, not directly to coenzyme Q.
E. Transports ATP to the cytoplasm. Incorrect: ATP is transported to the cytoplasm by the ATP/ADP translocase, not by coenzyme Q. Coenzyme Q is involved in electron transport, not ATP movement.
16. An unskilled worker at a water garden and plant nursery was tasked with cleaning up a spill of white powder in the storage shed. He was later found with labored breathing and convulsions. Upon investigation, the powder was identified as rotenone, a known pesticide. Rotenone exposure induces respiratory distress by inhibiting a complex of the electron transport chain (ETC). Which of the following best describes the process inhibited by rotenone?
A. Electron transfer from cytochrome a1/a3 to oxygen
B. Electron transfer from NADH to coenzyme Q
C. Oxidation of coenzyme Q
D. Reduction of cytochrome c
E. Electron transfer from cytochrome c to cytochrome a1/a3
Correct Option: B. Electron transfer from NADH to coenzyme Q. Rotenone inhibits Complex I (NADH-CoQ reductase) in the electron transport chain. This complex is responsible for the transfer of electrons from NADH to ubiquinone (coenzyme Q). When Complex I is inhibited, electron flow through the ETC is blocked, reducing ATP production and leading to symptoms such as respiratory distress and convulsions due to energy deficiency in critical tissues.
Incorrect Options:
A. Electron transfer from cytochrome a1/a3 to oxygen. Incorrect: This step occurs at Complex IV (cytochrome c oxidase), which transfers electrons to molecular oxygen. This process is inhibited by cyanide, not rotenone.
C. Oxidation of coenzyme Q. Incorrect: Coenzyme Q passes electrons to Complex III (cytochrome bc1 complex). Rotenone inhibits the electron transfer to coenzyme Q, not its oxidation.
D. Reduction of cytochrome c. Incorrect: Cytochrome c is reduced by electrons from Complex III, not Complex I, which is the target of rotenone.
E. Electron transfer from cytochrome c to cytochrome a1/a3. Incorrect: This occurs at Complex IV, which is involved in the final steps of the ETC and is not affected by rotenone.
17. A 42-year-old factory worker is rushed to the emergency department after accidental exposure to cyanide gas. He presents with rapid breathing, confusion, and metabolic acidosis. Cyanide inhibits oxidative phosphorylation by blocking the electron transport chain. Which of the following is most likely to increase in response to cyanide exposure?
A. Gluconeogenesis to provide more glucose for metabolism
B. Transport of ADP into the mitochondria
C. Utilization of fatty acids to augment glucose utilization
D. Utilization of ketone bodies for energy generation
E. Lactic acid in the blood causing acidosis
Correct Option: E. Lactic acid in the blood causing acidosis. Cyanide blocks Complex IV (cytochrome c oxidase), inhibiting oxidative phosphorylation and preventing ATP production through aerobic respiration. As a result, cells rely on anaerobic glycolysis for energy, leading to the accumulation of lactate. This buildup of lactic acid in the blood causes metabolic acidosis, which is a hallmark of cyanide poisoning.
Incorrect Options:
A. Gluconeogenesis to provide more glucose for metabolism. Incorrect: While gluconeogenesis provides glucose in fasting states, it is not directly stimulated by cyanide exposure. The shift toward anaerobic glycolysis occurs without the need for increased gluconeogenesis.
B. Transport of ADP into the mitochondria. Incorrect: Cyanide inhibits the electron transport chain, so ADP transport into mitochondria will not increase, as oxidative phosphorylation is halted.
C. Utilization of fatty acids to augment glucose utilization. Incorrect: Fatty acid metabolism primarily relies on functional mitochondria for beta-oxidation and ATP production. In cyanide toxicity, oxidative phosphorylation is blocked, so fatty acid utilization is not enhanced.
D. Utilization of ketone bodies for energy generation. Incorrect: Ketone bodies are used during fasting and prolonged starvation, but they require functional mitochondria for ATP production, which is impaired in cyanide poisoning.
18. A 27-year-old male undergoing surgery for acute appendicitis receives halothane as an inhaled anesthetic. Shortly after induction, he develops hyperthermia, tachypnea, respiratory acidosis, and hyperkalemia, and the surgical team learns of a family history of similar events. The tentative diagnosis is malignant hyperthermia (MH), a life-threatening condition triggered by certain anesthetics. Which of the following processes is affected by halothane in malignant hyperthermia?
A. Inhibition of ADP/ATP transporter
B. Inhibition of NADH-Q oxidoreductase (Complex I)
C. Inhibition of Q-cytochrome c oxidoreductase (Complex III)
D. Inhibition of succinate Q reductase (Complex II)
E. Uncoupling of oxidative phosphorylation
Correct Option: E. Uncoupling of oxidative phosphorylation. In malignant hyperthermia, halothane triggers excessive calcium release from the sarcoplasmic reticulum, leading to sustained muscle contraction, heat production, and increased metabolism. This process results in the uncoupling of oxidative phosphorylation, where the electron transport chain continues, but ATP synthesis is impaired, and energy is dissipated as heat. This uncontrolled energy release causes hyperthermia and metabolic disturbances like acidosis and hyperkalemia.
Incorrect Options:
A. Inhibition of ADP/ATP transporter. Incorrect: The ADP/ATP transporter (adenine nucleotide translocase) exchanges ATP and ADP across the mitochondrial membrane. Halothane does not inhibit this transporter; atractyloside is a known inhibitor of this system.
B. Inhibition of NADH-Q oxidoreductase (Complex I). Incorrect: Rotenone inhibits Complex I, blocking the transfer of electrons from NADH to coenzyme Q. This mechanism is unrelated to malignant hyperthermia induced by halothane.
C. Inhibition of Q-cytochrome c oxidoreductase (Complex III). Incorrect: Complex III, involved in the transfer of electrons to cytochrome c, is not affected by halothane. Inhibitors of Complex III include antimycin A.
D. Inhibition of succinate Q reductase (Complex II). Incorrect: Complex II transfers electrons from succinate to coenzyme Q. Its inhibition is not related to malignant hyperthermia. Malonate is an example of a Complex II inhibitor.
19. A 45-year-old marathon runner visits a sports clinic complaining of fatigue during long runs. The physician explains that the rate of mitochondrial respiration is closely regulated to meet the body’s energy needs. The availability of which of the following molecules primarily controls the rate of mitochondrial respiration?
A. ADP
B. ATP
C. FAD
D. FMN
E. NAD⁺
Correct Option: A. ADP. The rate of mitochondrial respiration is tightly regulated by the availability of ADP in a process known as respiratory control. When ADP levels are high, the proton gradient drives the ATP synthase complex to produce ATP from ADP and inorganic phosphate, thus increasing oxygen consumption. Conversely, when ADP levels are low, respiration slows down, as there is no demand for ATP synthesis.
Incorrect Options:
B. ATP. Incorrect: ATP inhibits mitochondrial respiration when it accumulates, reducing the need for further ATP production. Thus, ATP availability slows respiration rather than increasing it.
C. FAD. Incorrect: FAD is a cofactor used in Complex II of the electron transport chain. While it participates in the electron transport process, it does not control the rate of mitochondrial respiration.
D. FMN. Incorrect: FMN is a cofactor for Complex I (NADH-CoQ oxidoreductase), but it is not a key factor regulating the overall rate of respiration.
E. NAD⁺. Incorrect: Although NAD⁺ is essential for the function of the citric acid cycle and electron transport chain, the availability of ADP is the primary factor that controls the rate of respiration.
20. Bongkrekic acid is a toxic compound that inhibits oxidative phosphorylation by blocking a critical step in the mitochondrial energy production pathway. Which of the following processes is inhibited by bongkrekic acid?
A. Inhibiting ADP/ATP transporter
B. Inhibiting NADH-Q oxidoreductase (Complex I)
C. Inhibiting Q-cytochrome c oxidoreductase (Complex III)
D. Inhibiting succinate Q reductase (Complex II)
E. Inhibiting cytochrome a1-a3 oxidase
Correct Option: A. Inhibiting ADP/ATP transporter: Bongkrekic acid inhibits the ADP/ATP translocase (adenine nucleotide translocase), which is essential for exchanging ATP and ADP across the inner mitochondrial membrane. This inhibition prevents the export of ATP to the cytosol and the import of ADP into the mitochondrial matrix, ultimately halting ATP synthesis and impairing cellular energy metabolism.
Incorrect Options:
B. Inhibiting NADH-Q oxidoreductase (Complex I): Incorrect: Complex I, inhibited by rotenone, transfers electrons from NADH to ubiquinone (CoQ). However, this is not the mechanism of bongkrekic acid toxicity.
C. Inhibiting Q-cytochrome c oxidoreductase (Complex III): Incorrect: Complex III transfers electrons from reduced CoQ to cytochrome c. This process is inhibited by antimycin A, not bongkrekic acid.
D. Inhibiting succinate Q reductase (Complex II): Incorrect: Complex II, which transfers electrons from succinate to CoQ, is inhibited by malonate, not bongkrekic acid.
E. Inhibiting cytochrome a1-a3 oxidase: Incorrect: Complex IV, also known as cytochrome c oxidase, transfers electrons to oxygen and is inhibited by cyanide and carbon monoxide, not bongkrekic acid.
