Library
Gluconeogenesis- Short-answer questions
- November 13, 2024
- Posted by: Namrata Chhabra
- Category: Energy metabolism Learning resources Library Metabolism of Carbohydrates Question Bank Question Bank Quick Revision Series Short-answer questions Short-Answer questions USMLE Content
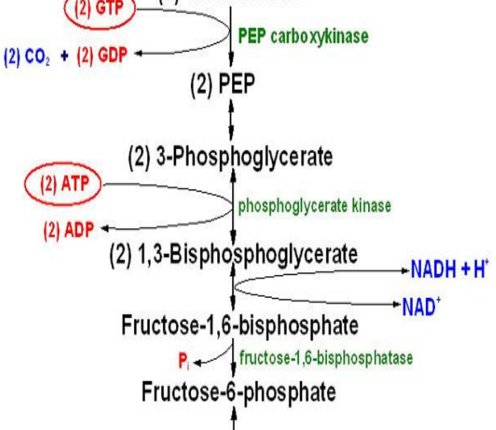
Question 1: Explain why gluconeogenesis is not simply the reverse of glycolysis.
Answer: Gluconeogenesis is not simply the reverse of glycolysis because there are three irreversible reactions in glycolysis that must be bypassed in gluconeogenesis. These irreversible reactions are catalyzed by hexokinase, phosphofructokinase, and pyruvate kinase. Gluconeogenesis uses different enzymes to bypass these steps, making it a distinct pathway.
Question 2: During a prolonged fast, why is gluconeogenesis the main source of glucose?
Answer: During a prolonged fast, glycogen stores are depleted, meaning the body can no longer rely on glycogenolysis to maintain blood glucose levels. Gluconeogenesis becomes the primary source of glucose because it can synthesize glucose from non-carbohydrate precursors such as amino acids, lactate, glycerol, and propionate.
Question 3: Why does the first bypass reaction in gluconeogenesis require two enzymes?
Answer: The first bypass reaction, converting pyruvate to phosphoenolpyruvate, requires two enzymes: pyruvate carboxylase and phosphoenolpyruvate carboxykinase. This is because the direct conversion of pyruvate to phosphoenolpyruvate is thermodynamically unfavorable. Pyruvate carboxylase first converts pyruvate to oxaloacetate in the mitochondria, which is then transported to the cytoplasm and converted to phosphoenolpyruvate by phosphoenolpyruvate carboxykinase.
Question 4: Explain the role of the Cori cycle in maintaining blood glucose levels during exercise.
Answer: During intense exercise, muscle cells produce lactate through anaerobic glycolysis. The Cori cycle helps to recycle this lactate back into glucose. Lactate is transported to the liver, where it is converted to glucose through gluconeogenesis. This glucose is then released back into the bloodstream, providing a source of energy for the muscles and replenishing glycogen stores.
Question 5: What is the significance of biotin in gluconeogenesis, and what happens in its deficiency?
Answer: Biotin is a coenzyme for pyruvate carboxylase, a key enzyme in the first bypass reaction of gluconeogenesis. Biotin deficiency impairs pyruvate carboxylase activity, hindering gluconeogenesis. This can lead to hypoglycemia due to the reduced ability to synthesize glucose from non-carbohydrate sources. Biotin deficiency can occur from consuming large amounts of raw egg whites, as avidin in raw egg whites binds to biotin and prevents its absorption9.
Question 6: Explain why fatty acid oxidation can stimulate gluconeogenesis.
Answer: Fatty acid oxidation produces acetyl CoA, which is an allosteric activator of pyruvate carboxylase, a key enzyme in gluconeogenesis. Acetyl CoA also inhibits pyruvate dehydrogenase, the enzyme that converts pyruvate to acetyl CoA in the mitochondria. This shift in metabolism favors gluconeogenesis by increasing pyruvate availability for glucose synthesis.
Question 7: Why is alcohol consumption associated with hypoglycemia, particularly in malnourished individuals?
Answer: Alcohol consumption can lead to hypoglycemia, especially in malnourished individuals, due to several factors. Alcohol metabolism alters the NAD+/NADH ratio in the liver, favoring the conversion of pyruvate to lactate. This depletes pyruvate, a key substrate for gluconeogenesis. Alcohol also inhibits fatty acid oxidation, reducing the availability of acetyl CoA, an activator of gluconeogenesis. Additionally, chronic alcohol abuse can deplete hepatic glycogen stores, further contributing to hypoglycemia.
Question 8: How does the regulation of gluconeogenesis differ between the well-fed state and the fasting state?
Answer: In the well-fed state, insulin levels are high, promoting glycolysis and inhibiting gluconeogenesis. Insulin stimulates the expression of key glycolytic enzymes and suppresses the expression of gluconeogenic enzymes. During fasting, glucagon levels rise, stimulating gluconeogenesis and inhibiting glycolysis. Glucagon promotes the expression of gluconeogenic enzymes and inhibits the expression of glycolytic enzymes. This hormonal regulation ensures that glucose production is tailored to the body’s energy needs.
Question 9: Why are premature and low-birth-weight infants more susceptible to hypoglycemia?
Answer: Premature and low-birth-weight infants are more susceptible to hypoglycemia because they have limited glycogen stores and immature gluconeogenic enzymes. They also have little adipose tissue to provide alternative fuels like free fatty acids and ketone bodies. This makes them highly reliant on a constant supply of glucose, and any disruption in glucose production or intake can quickly lead to hypoglycemia.
Question 10: Describe the role of the glucose-alanine cycle in maintaining blood glucose during fasting.
Answer: The glucose-alanine cycle helps maintain blood glucose during fasting by providing a mechanism for the liver to produce glucose from amino acids released from muscle protein breakdown. In this cycle, muscle protein is broken down into amino acids, and some are converted to alanine. Alanine is transported to the liver, where it is deaminated to pyruvate, which is then used for gluconeogenesis. The glucose produced is released into the bloodstream, providing energy for various tissues, including the brain. This cycle also plays a role in nitrogen disposal. The amino group removed from alanine in the liver is converted to urea and excreted in the urine, preventing the buildup of toxic ammonia.
Question 11: What are the major substrates of gluconeogenesis, and how are they categorized based on their metabolic origin?
Answer: There are four major substrates of gluconeogenesis:
○ Glucogenic amino acids: These amino acids, derived from protein breakdown, can be converted into pyruvate or intermediates of the citric acid cycle. All amino acids except leucine are glucogenic.
○ Lactate: Lactate, produced by anaerobic glycolysis in exercising muscle or red blood cells, is readily converted to pyruvate.
○ Glycerol: This is released during the breakdown of triglycerides in adipose tissue.
○ Propionate: This short-chain fatty acid is produced primarily in ruminants but also arises from certain metabolic processes in humans.
Question 12: Describe how lactate enters the gluconeogenic pathway and the physiological cycle associated with this process.
Answer: Lactate produced in exercising muscle or red blood cells is transported to the liver, where it is converted to pyruvate by the enzyme lactate dehydrogenase. This pyruvate then enters the gluconeogenic pathway. This process is part of the Cori cycle, which involves the continuous cycling of lactate and glucose between the muscle and liver5.
Question 13: Explain the role of glycerol as a gluconeogenic substrate, its entry point into the pathway, and its importance during fasting.
Answer: Glycerol, released from the breakdown of triglycerides in adipose tissue, enters gluconeogenesis at the level of dihydroxyacetone phosphate (DHAP). This molecule is an intermediate in both glycolysis and gluconeogenesis. During fasting, glycerol becomes a crucial substrate for gluconeogenesis as triglycerides are broken down to provide energy.
Question 14: Discuss the entry point of propionate into gluconeogenesis and its relevance in different organisms.
Answer: Propionate enters the gluconeogenic pathway via the citric acid cycle. It is converted to succinyl CoA, which is an intermediate in the cycle. While propionate is a major glucose precursor in ruminants, it plays a relatively minor role in human gluconeogenesis.
Question 15: Why is it significant that glycerol kinase is absent in adipose tissue, and what implications does this have for glycerol metabolism during fasting?
Answer: The absence of glycerol kinase in adipose tissue means that glycerol released from triglyceride breakdown cannot be re-esterified back into triglycerides within the adipose tissue. Consequently, glycerol is released into the circulation and transported to the liver, where it is primarily used for gluconeogenesis during fasting conditions.
Question 16: A patient presents with fasting hypoglycemia. Biochemical analysis reveals a deficiency in pyruvate carboxylase. Explain the rationale of hypoglycemia in this patient.
Answer: Pyruvate carboxylase catalyzes the first step in the conversion of pyruvate to phosphoenolpyruvate, a crucial step in gluconeogenesis. This reaction occurs in the mitochondria and involves the carboxylation of pyruvate to oxaloacetate using biotin as a coenzyme. A deficiency in pyruvate carboxylase impairs the ability to convert pyruvate into oxaloacetate, thereby hindering the production of glucose from non-carbohydrate sources. This leads to a reduction in hepatic glucose production, contributing to hypoglycemia, especially during periods of fasting when gluconeogenesis is essential for maintaining blood glucose levels.
Question 17: An individual on a high-protein, low-carbohydrate diet experiences increased gluconeogenesis. Explain how this dietary pattern affects the regulation of gluconeogenesis, considering both allosteric and hormonal mechanisms.
Answer: A high-protein, low-carbohydrate diet leads to an increased reliance on gluconeogenesis for glucose production. The abundance of amino acids from the high-protein intake provides substrates for gluconeogenesis. The low-carbohydrate intake results in reduced insulin and elevated glucagon levels. Glucagon stimulates the expression of phosphoenolpyruvate carboxykinase and fructose 1,6-bisphosphatase, key gluconeogenic enzymes. Furthermore, the breakdown of amino acids and fatty acids generates acetyl-CoA, an allosteric activator of pyruvate carboxylase, further promoting gluconeogenesis.
Question 18: Explain why a deficiency in biotin can lead to impaired gluconeogenesis and potentially cause hypoglycemia, emphasizing the specific role of biotin in the pathway.
Answer: Biotin is a crucial coenzyme for pyruvate carboxylase, the enzyme responsible for converting pyruvate to oxaloacetate in the first bypass step of gluconeogenesis. Biotin acts as a carrier of carbon dioxide, facilitating its addition to pyruvate. In biotin deficiency, the activity of pyruvate carboxylase is impaired, hindering the conversion of pyruvate to oxaloacetate, which is a key precursor for phosphoenolpyruvate synthesis. This disruption in the gluconeogenic pathway limits the liver’s ability to produce glucose from non-carbohydrate sources, potentially leading to hypoglycemia, especially during fasting when blood glucose levels rely heavily on gluconeogenesis.
Question 19: Compare and contrast the regulation of gluconeogenesis by glucagon and insulin, highlighting their respective effects on key enzymes and the overall pathway.
Answer: Glucagon and insulin exert opposing effects on gluconeogenesis. Glucagon, released during fasting, stimulates gluconeogenesis by promoting the expression of key gluconeogenic enzymes, including phosphoenolpyruvate carboxykinase and fructose 1,6-bisphosphatase4. In contrast, insulin, released after a meal, inhibits gluconeogenesis by stimulating the expression of glycolytic enzymes and suppressing the expression of gluconeogenic enzymes. This reciprocal regulation by glucagon and insulin ensures that glucose production is appropriately adjusted to maintain blood glucose homeostasis in response to changes in nutritional status.
Question 20: A sprinter experiences muscle fatigue during a race. Explain the role of the Cori cycle in this scenario, detailing the metabolic processes in both muscle and liver.
Answer: During intense exercise, muscle cells rely heavily on anaerobic glycolysis for energy production, leading to the accumulation of lactate. The Cori cycle plays a crucial role in removing lactate from the muscle and replenishing glucose levels. Lactate generated in the muscle is transported to the liver, where it is converted to pyruvate by lactate dehydrogenase. The liver then utilizes this pyruvate for gluconeogenesis, synthesizing glucose that can be released back into the bloodstream to fuel the working muscles and replenish glycogen stores. This cycle helps delay muscle fatigue by removing lactate, which can contribute to muscle acidosis, and providing a continuous supply of glucose for energy.
Question 21: An individual with chronic alcoholism is brought to the emergency room with severe hypoglycemia. Discuss the metabolic reasons behind alcohol-induced hypoglycemia, considering the effects of alcohol metabolism on gluconeogenesis.
Answer: Alcohol-induced hypoglycemia occurs due to the metabolic effects of alcohol on gluconeogenesis. Alcohol metabolism increases the NADH/NAD+ ratio in the liver, favoring the conversion of pyruvate to lactate, which depletes pyruvate, a key substrate for gluconeogenesis. Elevated NADH levels also inhibit fatty acid oxidation, reducing the availability of acetyl-CoA, an allosteric activator of pyruvate carboxylase. Furthermore, chronic alcohol abuse can deplete hepatic glycogen stores, further compromising the body’s ability to maintain blood glucose levels.
Question 22: A patient with type 1 diabetes presents with diabetic ketoacidosis. Explain how the lack of insulin in this condition contributes to increased gluconeogenesis and hyperglycemia.
Answer: In type 1 diabetes, the absence of insulin leads to uncontrolled gluconeogenesis and hyperglycemia. Without insulin, glucagon levels remain elevated, stimulating the expression of gluconeogenic enzymes and promoting glucose production. The lack of insulin also prevents the suppression of gluconeogenic enzymes, further exacerbating glucose overproduction. Additionally, the breakdown of fatty acids for energy in the absence of insulin generates acetyl-CoA, which activates pyruvate carboxylase and further fuels gluconeogenesis.
Question 23: Describe the role of fructose 2,6-bisphosphate in the regulation of gluconeogenesis, specifically addressing its influence on key enzymes and the overall pathway.
Answer: Fructose 2,6-bisphosphate is a potent allosteric regulator of both glycolysis and gluconeogenesis. It activates phosphofructokinase-1 (PFK-1), a key enzyme in glycolysis, while inhibiting fructose 1,6-bisphosphatase, a key enzyme in gluconeogenesis. Insulin stimulates the production of fructose 2,6-bisphosphate, promoting glycolysis. Conversely, glucagon inhibits fructose 2,6-bisphosphate production, favoring gluconeogenesis. This regulation ensures that glycolysis and gluconeogenesis are reciprocally controlled to maintain glucose homeostasis.
Question 24: Explain why prolonged fasting leads to increased reliance on amino acids as substrates for gluconeogenesis.
Answer: During prolonged fasting, glycogen stores become depleted, and the body must rely increasingly on gluconeogenesis to maintain blood glucose levels. As fasting progresses, amino acids derived from muscle protein breakdown become the primary substrate for gluconeogenesis. The breakdown of muscle protein releases amino acids, many of which can be converted into pyruvate or intermediates of the citric acid cycle, providing precursors for glucose synthesis. This process helps sustain blood glucose levels but also results in muscle wasting during prolonged fasting.
Question 25: Why is gluconeogenesis considered an energetically expensive process?
Answer: Gluconeogenesis requires the hydrolysis of six nucleotide triphosphate molecules to synthesize one molecule of glucose from pyruvate. In contrast, glycolysis, which converts glucose to pyruvate, only generates two ATP molecules (anaerobically). Therefore, gluconeogenesis is not simply a reversal of glycolysis but a distinct pathway requiring substantial energy input.
Question 26: What is the source of GTP utilized by phosphoenolpyruvate carboxykinase in gluconeogenesis, and why is this significant?
Answer: The GTP used by phosphoenolpyruvate carboxykinase in the liver and kidney is produced by succinate thiokinase in the citric acid cycle. This compartmentalization of GTP production in these gluconeogenic tissues highlights a metabolic adaptation that efficiently couples energy generation with glucose synthesis.
Question 27: Explain the role of fatty acid oxidation in supporting gluconeogenesis.
Answer: Acetyl-CoA, the product of fatty acid oxidation, allosterically activates pyruvate carboxylase, a key enzyme in the first bypass step of gluconeogenesis. This activation promotes glucose synthesis when fatty acid breakdown is increased, such as during fasting, ensuring a supply of glucose for tissues that rely on it for energy. ATP produced during fatty acid oxidation also helps glucose synthesis.
Question 28: Considering the energy cost of gluconeogenesis, why doesn’t the body simply rely solely on glycogen stores for glucose during fasting?
Answer: While the liver glycogen can meet glucose demands for less than 24 hours in the absence of dietary carbohydrates, glycogen stores are finite. Prolonged fasting depletes glycogen stores, necessitating gluconeogenesis. The body must balance using readily available glycogen with the need to conserve it for crucial situations. Gluconeogenesis allows the body to synthesize glucose from non-carbohydrate sources, sparing glycogen and extending the period the body can maintain blood glucose levels without food intake.
Question 29: What are the implications of impaired gluconeogenesis for energy metabolism in various tissues, particularly the brain and red blood cells?
Answer: Gluconeogenesis is essential for providing glucose to tissues that rely on it as their primary energy source. The brain and red blood cells heavily depend on glucose. The brain has limited capacity to use alternative fuels, and red blood cells lack mitochondria, making them exclusively dependent on glucose for energy. Impaired gluconeogenesis, as seen in certain metabolic disorders or deficiencies, can lead to a shortage of glucose supply to these tissues. This can result in hypoglycemia, causing brain dysfunction, which can lead to coma and death.
Question 30: In a chronic alcoholic with a biotin deficiency, which conversion is Impaired, causing hypoglycemia in a fasting state?
Answer: In a chronic alcoholic with a biotin deficiency, the conversion of pyruvate to oxaloacetate is impaired, contributing to hypoglycemia in a fasting state. Here’s why:
Biotin’s Role: Biotin is a crucial coenzyme for pyruvate carboxylase, a key enzyme in gluconeogenesis. Pyruvate carboxylase catalyzes the carboxylation of pyruvate to oxaloacetate in the mitochondria. This reaction is the first step in the first bypass of gluconeogenesis, essential for overcoming the irreversible reaction of pyruvate kinase in glycolysis1.
Biotin Deficiency: A deficiency in biotin directly impairs the activity of pyruvate carboxylase. This impairment slows down the conversion of pyruvate to oxaloacetate, hindering the first bypass step of gluconeogenesis.
Chronic Alcoholism and Gluconeogenesis: Chronic alcohol abuse further exacerbates the situation. Alcohol metabolism increases the NADH/NAD+ ratio in the liver. This high NADH/NAD+ ratio favors the conversion of pyruvate to lactate, further depleting the pyruvate pool available for gluconeogenesis3.
Impact on Fasting State: During fasting, the body relies heavily on gluconeogenesis to maintain blood glucose levels as glycogen stores become depleted. With impaired pyruvate carboxylase activity due to biotin deficiency and the shift towards lactate production due to alcoholism, the liver’s ability to produce glucose from non-carbohydrate sources is severely compromised. This ultimately leads to hypoglycemia.
Importance of Pyruvate to Oxaloacetate Conversion: The conversion of pyruvate to oxaloacetate is not only crucial for gluconeogenesis, but it also replenishes oxaloacetate in the citric acid cycle. This replenishment is vital for the continued function of the citric acid cycle, which is a central hub of energy metabolism.
In summary, the combined effects of biotin deficiency and chronic alcohol abuse create a metabolic bottleneck in the conversion of pyruvate to oxaloacetate. This bottleneck cripples gluconeogenesis, making it difficult for the body to maintain blood glucose levels during fasting and ultimately leading to hypoglycemia.
Question 31: How does the end product of gluconeogenesis differ in liver and skeletal muscle?
Answer: The end product of gluconeogenesis, glucose 6-phosphate, has different fates in the liver and skeletal muscle. This difference stems from the presence or absence of a specific enzyme.
Liver: The liver expresses glucose-6-phosphatase, an enzyme located in the endoplasmic reticulum. This enzyme catalyzes the hydrolysis of glucose 6-phosphate to free glucose, which can then be released into the bloodstream. This ability is crucial because the liver plays a central role in maintaining blood glucose homeostasis, providing glucose for other tissues, especially during fasting.
Skeletal Muscle: Skeletal muscle lacks glucose-6-phosphatase. Consequently, the glucose 6-phosphate produced by gluconeogenesis in muscle cannot be converted to free glucose. Instead, it’s primarily used for glycogen synthesis, replenishing the muscle’s glycogen stores. This difference highlights the distinct roles of the liver and muscle in glucose metabolism. The liver functions as a glucose provider for the body, while muscle primarily uses glucose for its own energy needs and stores excess glucose as glycogen.
Question 32- Which enzyme is inhibited during a low-energy state and by a high-carbohydrate diet but is activated during fasting and by the product of even-chain fatty acid oxidation?
Answer: The enzyme pyruvate carboxylase is inhibited during a low-energy state and by a high-carbohydrate diet, but it is activated during fasting and by the product of even-chain fatty acid oxidation.
Pyruvate Carboxylase’s Role: Pyruvate carboxylase catalyzes the conversion of pyruvate to oxaloacetate, a crucial first step in gluconeogenesis. This reaction occurs in the mitochondria and requires biotin as a coenzyme.
Inhibition by Low-Energy State and High-Carbohydrate Diet: During a low-energy state, high levels of AMP signal low cellular energy, inhibiting pyruvate carboxylase. Similarly, a high-carbohydrate diet leads to elevated insulin levels, which also represses pyruvate carboxylase expression. These conditions favor glycolysis for energy production from readily available glucose, making gluconeogenesis less necessary.
Activation During Fasting and by Fatty Acid Oxidation: During fasting, glucagon levels rise, stimulating the production of pyruvate carboxylase. Even-chain fatty acid oxidation produces acetyl-CoA, an allosteric activator of pyruvate carboxylase. This activation boosts the conversion of pyruvate to oxaloacetate, fueling gluconeogenesis when glucose from dietary sources is limited.
Key Concepts:
- Metabolic Regulation: The regulation of pyruvate carboxylase exemplifies the intricate control mechanisms that govern metabolic pathways. These mechanisms ensure that the body efficiently utilizes available energy sources and synthesizes glucose when needed.
- Hormonal Control: Glucagon and insulin, key metabolic hormones, play a crucial role in regulating pyruvate carboxylase activity. Glucagon promotes gluconeogenesis during fasting, while insulin favors glycolysis in the well-fed state.
- Allosteric Modulation: Acetyl-CoA, a product of fatty acid oxidation, acts as an allosteric activator of pyruvate carboxylase, demonstrating how metabolic intermediates directly influence enzyme activity. This activation highlights the interconnectedness of metabolic pathways and the body’s ability to adapt to different fuel sources.
Question 33- Which enzyme generates a precursor for gluconeogenesis during intense muscle activity, and what is the product of this reaction?
Answer: The enzyme lactate dehydrogenase catalyzes the reaction, converting pyruvate to lactate during anaerobic glycolysis in muscle. Lactate is the precursor for gluconeogenesis, as it is transported to the liver and converted back to glucose through the Cori cycle.
Question 34- What alternative reaction can produce the product of a biotin-dependent enzyme in cases of biotin deficiency or enzyme dysfunction?
Answer: If the biotin-dependent enzyme pyruvate carboxylase is defective or there is a biotin deficiency, the product oxaloacetate can be alternatively produced through the transamination of aspartate by the enzyme aspartate aminotransferase (AST). This reaction converts aspartate and α-ketoglutarate into oxaloacetate and glutamate.
Question 35- How does the conversion of glycerol to glycerol-3-phosphate support survival during starvation?
Brief Answer: During starvation, glycerol released from triglyceride breakdown in adipose tissue is converted to glycerol-3-phosphate by the enzyme glycerol kinase in the liver. Glycerol-3-phosphate is then oxidized to dihydroxyacetone phosphate (DHAP), which enters gluconeogenesis to produce glucose. This process helps maintain blood glucose levels, ensuring a critical energy supply for glucose-dependent tissues like the brain and red blood cells.
Question 36- Which enzymes are upregulated and downregulated in the muscle cells of a sprinter?
Answer- In the muscle cells of a sprinter:
- Upregulated enzyme: Phosphofructokinase-1 (PFK-1), which drives glycolysis to rapidly generate ATP. Glycogen phosphorylase is also upregulated to mobilize glycogen stores for energy.
- Downregulated enzyme: Enzymes in the citric acid cycle may be downregulated as anaerobic glycolysis predominates over oxidative metabolism during intense, short bursts of activity.
In the muscle cells of a sprinter, gluconeogenesis enzymes are not highly active because muscle tissue does not perform gluconeogenesis. Instead, the liver handles this process during recovery to replenish blood glucose. However, glycolytic enzymes like pyruvate kinase and PFK-1 are upregulated in muscles for rapid ATP generation, and lactate dehydrogenase becomes active to convert pyruvate to lactate during anaerobic conditions.
Question 37- What happens to peripheral glucose uptake, glucose utilization, adipolysis, and fatty acid oxidation enzymes after a carbohydrate-rich meal following a 24-hour fast?
Answer: After consuming a carbohydrate-rich meal following a 24-hour fast:
- Peripheral glucose uptake: Increases due to insulin release, which stimulates glucose uptake by insulin-sensitive tissues like muscle and adipose tissue through GLUT4 transporters.
- Glucose utilization: Increases as insulin activates glycolysis and glycogenesis to store glucose as glycogen in the liver and muscles.
- Adipolysis: Decreases because insulin inhibits hormone-sensitive lipase (HSL), reducing the breakdown of triglycerides in adipose tissue.
- Fatty acid oxidation-related enzymes: Downregulated as insulin suppresses fatty acid oxidation by inhibiting carnitine palmitoyltransferase-1 (CPT-1), a key enzyme in transporting fatty acids into mitochondria for oxidation. Instead, glucose becomes the primary energy source.
Question 38- How does vitamin B6 deficiency affect gluconeogenesis?
Answer- Vitamin B6, in its active form, pyridoxal phosphate (PLP), is a cofactor for transaminases critical for gluconeogenesis. A deficiency. Transaminases convert amino acids into gluconeogenic intermediates (e.g., alanine to pyruvate and aspartate to oxaloacetate). Without adequate B6, the supply of these intermediates is reduced.
