Library
Glycolysis Review- NBME style questions with complete explanations
- October 21, 2024
- Posted by: Namrata Chhabra
- Category: Energy metabolism Learning resources Library Metabolism of Carbohydrates Multiple-choice questions Multiple-choice questions PowerPoint presentations Practice questions Question Bank Question Bank Quick Revision Series USMLE Content USMLE Style questions USMLE styled question bank
Q.1 Identify the Phosphoester that is predominantly encountered in erythrocytes and plays a vital role in modulating the oxygen-binding affinity of hemoglobin:
A. 1,3-Bisphosphoglycerate
B. 2,3-Bisphosphoglycerate
C. Fructose 1,6-bisphosphate
D. Fructose 6-phosphate
E. Glucose 6-phosphate
The correct answer is B. 2,3-Bisphosphoglycerate (2,3-BPG): 2,3-Bisphosphoglycerate is a negatively charged molecule found in high concentrations within erythrocytes. 2,3-BPG binds with greater affinity to deoxygenated hemoglobin than to oxygenated hemoglobin, thus stabilizing the deoxygenated form and promoting the release of oxygen. This effect is crucial for enhancing oxygen delivery to tissues that require it the most, such as actively respiring muscles. The presence and concentration of 2,3-BPG significantly influence hemoglobin’s oxygen-binding affinity, serving as an essential regulator of oxygen release from hemoglobin.
Incorrect Options:
A. 1,3-Bisphosphoglycerate: Although 1,3-Bisphosphoglycerate is an intermediate in glycolysis, it does not play a direct role in modulating the oxygen-binding affinity of hemoglobin. Instead, it is involved in the metabolic pathway leading to ATP production.
C. Fructose 1,6-bisphosphate: Fructose 1,6-bisphosphate is an important intermediate in glycolysis, but it does not directly affect the oxygen-binding affinity of hemoglobin. It is involved in the split of glucose into two three-carbon sugars, which are further processed in glycolysis.
D. Fructose 6-phosphate: Fructose 6-phosphate is another glycolytic intermediate that does not have a direct role in the regulation of hemoglobin’s oxygen affinity. Its main role is as a precursor to fructose 1,6-bisphosphate in the glycolytic pathway.
E. Glucose 6-phosphate: Glucose 6-phosphate is the product of the first step in glycolysis, where glucose is phosphorylated. Like the other incorrect options, it does not directly influence hemoglobin’s oxygen-binding properties. Its primary significance lies in glycolysis and glucose metabolism.
The specific interaction of 2,3-BPG with hemoglobin exemplifies the intricate regulatory mechanisms that control oxygen delivery in the human body, highlighting the importance of biochemical compounds in physiological processes.
Q. 2-Which sugar among the following is characterized by having a significantly lower Km value with hexokinase, indicating a higher affinity between the enzyme and this specific substrate?
A. Fructose
B. Galactose
C. Glucose
D. Glycerol
E. Mannose
The correct answer is C. Glucose: Hexokinase is known to have a high affinity for glucose, making it the primary substrate for the enzyme in most body tissues.
Incorrect Options:
A. Fructose: While hexokinase can phosphorylate fructose, it has a higher Km for fructose than glucose, indicating a lower affinity for this sugar.
B. Galactose: Galactose can be phosphorylated by hexokinase, but it is not the preferred substrate, and it has a higher Km compared to glucose, showing lower affinity.
D. Glycerol: Glycerol is not a substrate for hexokinase. The enzyme that phosphorylates glycerol is glycerol kinase, so hexokinase’s Km for glycerol is not applicable.
E. Mannose: Mannose can be phosphorylated by hexokinase, but it has a higher Km value compared to glucose, indicating that hexokinase has a lower affinity for mannose than for glucose.
Q.3- Identity which ‘one’ of the following compounds is not recognized as an intermediate in the glycolytic pathway.
A. Dihydroxyacetone phosphate
B. Fructose 1, 6 bisphosphate
C. Fructose 6 phosphate
D. Glucose 6 phosphate
E. Glycerol 3 phosphate
The correct answer is E. Glycerol 3-phosphate. Glycerol 3-phosphate is involved in lipid metabolism, particularly in triglyceride synthesis and the glycerol phosphate shuttle used for transferring electrons to mitochondria. However, it is not a part of glycolysis and does not appear as an intermediate in this metabolic pathway.
Incorrect Options:
A. Dihydroxyacetone phosphate (DHAP) is a recognized glycolytic intermediate. It forms from the cleavage of fructose 1,6-bisphosphate and can be converted into glyceraldehyde-3-phosphate, another key glycolytic molecule.
B. Fructose 1,6-bisphosphate is also part of glycolysis. It is produced through the phosphorylation of fructose 6-phosphate by the enzyme phosphofructokinase-1, which is a crucial regulatory step in the glycolytic pathway.
C. Fructose 6-phosphate is an intermediate formed from glucose 6-phosphate. It undergoes further phosphorylation to become fructose 1,6-bisphosphate, continuing through the glycolytic sequence.
D. Glucose 6-phosphate is the first intermediate formed in glycolysis when glucose is phosphorylated by hexokinase or glucokinase. This molecule can enter multiple pathways, including glycolysis and the pentose phosphate pathway.
Since glycerol 3-phosphate is not involved in glycolysis, it is the only correct answer among the given options.
Q.4- Upon entering the cytoplasm, glucose becomes an important substrate for various metabolic pathways. Identify which ‘one’ of the following enzymatic conversions serves as a committal step, predominantly directing glucose toward its oxidative breakdown via the glycolytic pathway?
A. Fructose 6-P to Fructose 1,6 bisphosphate
B. Fructose 6-P to Fructose 2,6 bisphosphate
C. Glucose 6-P to Fructose 6-P
D. Glucose 6-P to Glucose 1-P
E. Glucose to Glucose 6-P
The correct answer is A. Fructose 6-P to Fructose 1,6-bisphosphate.
This step is catalyzed by the enzyme phosphofructokinase-1 (PFK-1), which is considered the committed and rate-limiting step of glycolysis. Once fructose 6-phosphate is converted to fructose 1,6-bisphosphate, the pathway is irreversibly committed to proceeding through glycolysis, ultimately leading to the oxidative breakdown of glucose into pyruvate.
Incorrect Options:
B. Fructose 6-P to Fructose 2,6-bisphosphate is not part of glycolysis. Fructose 2,6-bisphosphate is a regulatory molecule that activates PFK-1, enhancing the glycolytic flux, but it is not itself an intermediate in the glycolytic pathway.
C. Glucose 6-P to Fructose 6-P is a reversible step in glycolysis catalyzed by phosphoglucoisomerase. However, this step is not considered a commitment point since the glucose 6-phosphate can still enter other metabolic pathways, such as the pentose phosphate pathway or glycogenesis.
D. Glucose 6-P to Glucose 1-P is involved in glycogen metabolism. This step channels glucose towards glycogen synthesis or mobilization but is not directly related to glycolysis.
E. Glucose to Glucose 6-P is the first step of glycolysis, catalyzed by hexokinase or glucokinase. However, this step is not the committed step because glucose 6-phosphate can enter other pathways, such as glycogen synthesis or the pentose phosphate pathway.
Thus, the conversion of fructose 6-phosphate to fructose 1,6-bisphosphate by PFK-1 is the key committed step that ensures glucose is directed toward glycolysis.
Q.5- Mature erythrocytes crucially rely on energy production to sustain ion gradients across their cell membranes, an important factor preventing cellular swelling and potential hemolytic anemia. Identify which ‘one’ of the following metabolic processes predominantly facilitates this energy generation, ensuring erythrocyte viability and function?
A. Fatty acid oxidation
B. Oxidation of glucose to CO2 and H2O
C. Oxidative phosphorylation
D. Substrate-level phosphorylation
E. TCA cycle
The correct answer is D. Substrate-level phosphorylation.
Mature erythrocytes lack mitochondria, which prevents them from using processes like oxidative phosphorylation or the TCA cycle for energy production. Instead, they rely solely on substrate-level phosphorylation occurring within the glycolytic pathway to generate ATP. This ATP is essential for maintaining ion gradients across the erythrocyte membrane, preventing cellular swelling and hemolytic anemia.
Incorrect Options:
A. Fatty acid oxidation does not occur in erythrocytes because they lack mitochondria, where β-oxidation of fatty acids takes place.
B. Oxidation of glucose to CO₂ and H₂O happens in cells with functional mitochondria via aerobic respiration. Erythrocytes, being devoid of mitochondria, cannot fully oxidize glucose to CO₂ and water.
C. Oxidative phosphorylation is a mitochondrial process in which ATP is produced from the electron transport chain. Since erythrocytes lack mitochondria, they cannot rely on this process for energy production.
E. TCA cycle (Krebs cycle) is a mitochondrial process involved in the complete oxidation of acetyl-CoA to CO₂. Erythrocytes cannot utilize this cycle as they do not possess mitochondria.
Thus, substrate-level phosphorylation, primarily via glycolysis, is the main source of ATP for mature erythrocytes, ensuring the maintenance of ion gradients and proper cell function.
Q.6- Within the multi-step process of glycolysis, certain enzymatic reactions are classified as irreversible under physiological conditions. Identify which ‘one’ of the following enzyme-catalyzed reactions does not exhibit this irreversible characteristic within the glycolytic pathway?
A. 3-phosphoglycerate kinase
B. Glucokinase
C. Hexokinase
D. Phosphofructokinase-1
E. Pyruvate kinase
The correct answer is A. 3-phosphoglycerate kinase.: Within the glycolytic pathway, 3-phosphoglycerate kinase catalyzes the conversion of 1,3-bisphosphoglycerate to 3-phosphoglycerate, producing ATP in the process. This reaction is reversible under physiological conditions, meaning it can proceed in both directions based on the concentrations of reactants and products. As such, it is not classified as an irreversible step in glycolysis.
Incorrect Options:
B. Glucokinase and C. Hexokinase both catalyze the phosphorylation of glucose to glucose-6-phosphate, which is an irreversible step. These enzymes trap glucose inside the cell by adding a phosphate group, preventing glucose from leaving the cell. This is a key regulatory point in glycolysis.
D. Phosphofructokinase-1 (PFK-1) catalyzes the conversion of fructose-6-phosphate to fructose-1,6-bisphosphate, and this reaction is irreversible under physiological conditions. It is a major regulatory step and rate-limiting point in glycolysis, controlled by ATP, AMP, and fructose-2,6-bisphosphate levels.
E. Pyruvate kinase catalyzes the final step in glycolysis, converting phosphoenolpyruvate (PEP) to pyruvate, generating ATP. This reaction is also irreversible under normal conditions and marks the end of glycolysis, committing pyruvate to further metabolic processes.
Thus, the only enzyme in the given options that does not catalyze an irreversible reaction is 3-phosphoglycerate kinase, making it the correct answer.
Q.7- Glycolysis intricately maneuvers through a series of enzymatic reactions, with some enzymes tasked with the strategic cleavage of carbon-carbon bonds to further metabolic progression. Identify which ‘one’ of the following enzymes executes a carbon-carbon bond cleavage within the glycolytic pathway?
A. Aldolase
B. Enolase
C. Glyceraldehyde-3-P dehydrogenase
D. Phosphoglycerate kinase
E. Phosphoglycerate mutase
The correct answer is A. Aldolase.
Aldolase catalyzes the cleavage of the six-carbon sugar fructose 1,6-bisphosphate into two three-carbon molecules: glyceraldehyde-3-phosphate (G3P) and dihydroxyacetone phosphate (DHAP). This cleavage of the carbon-carbon bond is a key step in glycolysis, splitting the molecule into smaller units that can continue through the pathway.
Incorrect Options:
B. Enolase catalyzes the dehydration of 2-phosphoglycerate to phosphoenolpyruvate. It does not involve carbon-carbon bond cleavage but instead removes water to form an enol intermediate.
C. Glyceraldehyde-3-phosphate dehydrogenase catalyzes the oxidation and phosphorylation of glyceraldehyde-3-phosphate to 1,3-bisphosphoglycerate, with the production of NADH. This is an oxidation-reduction reaction, not a carbon-carbon bond cleavage.
D. Phosphoglycerate kinase catalyzes the substrate-level phosphorylation of ADP to ATP using 1,3-bisphosphoglycerate. It transfers a phosphate group but does not cleave carbon-carbon bonds.
E. Phosphoglycerate mutase converts 3-phosphoglycerate into 2-phosphoglycerate by shifting the phosphate group within the same molecule. This reaction involves intramolecular rearrangement, not bond cleavage.
Thus, aldolase is the enzyme responsible for the cleavage of a carbon-carbon bond, a pivotal reaction that splits fructose 1,6-bisphosphate into two three-carbon molecules essential for further glycolytic progression.
Q.8- In a hypothetical scenario where an embryo has a complete deficiency of pyruvate kinase, hindering the conversion of phosphoenolpyruvate (PEP) to pyruvate in glycolysis, how many net moles of ATP would be directly produced from glycolysis during the conversion of one mole of glucose to PEP?
A. 0
B. 1
C. 2
D. 3
E. 4
The correct answer is A. 0: In glycolysis, ATP is both consumed and produced through various steps. When pyruvate kinase is deficient, the final step (conversion of PEP to pyruvate) does not occur, meaning no additional ATP is produced beyond the earlier steps. Let’s break down the process leading up to PEP.
ATP Consumption:
1. Glucose → Glucose-6-phosphate
This step is catalyzed by hexokinase or glucokinase and consumes 1 ATP.
2. Fructose-6-phosphate → Fructose-1,6-bisphosphate
This step is catalyzed by phosphofructokinase-1 (PFK-1) and consumes 1 ATP.
3. Total ATP consumed: 2 ATP
ATP Production:
1. 1,3-bisphosphoglycerate → 3-phosphoglycerate
This step is catalyzed by phosphoglycerate kinase and produces 2 ATP (since each molecule of glucose generates 2 molecules of 1,3-bisphosphoglycerate).
Total ATP produced: 2 ATP
Net ATP Calculation:
• ATP produced: 2 ATP
• ATP consumed: 2 ATP
Thus, net ATP = 2 – 2 = 0.
Since the final step (PEP → pyruvate) is blocked due to pyruvate kinase deficiency, the additional ATP that would normally be produced by this step is not generated. Therefore, the overall net ATP production remains 0 for the conversion of glucose to PEP.
Even though 2 ATP are produced earlier, they are balanced out by the 2 ATP consumed in earlier steps, leaving no net gain of ATP.
Q.9- In the context of glycolysis, which of the following metabolic intermediates is likely to accumulate in cell-free extracts when fluoride is introduced?
A. 2-phosphoglycerate
B. Fructose 1,6-bisphosphate
C. Glucose 6-phosphate
D. Phosphoenolpyruvate
E. Pyruvate
The correct answer is A. 2-phosphoglycerate: Fluoride inhibits the enzyme enolase, which catalyzes the conversion of 2-phosphoglycerate (2-PG) to phosphoenolpyruvate (PEP). As a result, when fluoride is present, the reaction catalyzed by enolase is blocked, causing an accumulation of 2-phosphoglycerate upstream of this step.
Incorrect Options:
B. Fructose 1,6-bisphosphate: This intermediate appears earlier in glycolysis and is not directly affected by fluoride inhibition. Its conversion to glyceraldehyde-3-phosphate and dihydroxyacetone phosphate will proceed normally.
C. Glucose 6-phosphate: This is the product of the first step of glycolysis, catalyzed by hexokinase. It is not affected by enolase inhibition, and its levels remain regulated independently of fluoride action.
D. Phosphoenolpyruvate (PEP): Since enolase is inhibited by fluoride, the formation of PEP from 2-phosphoglycerate is blocked. Therefore, PEP levels would not increase and may even decrease.
E. Pyruvate: Pyruvate is the end product of glycolysis, but because the formation of PEP is hindered by fluoride, less pyruvate will be produced.
Conclusion:
The inhibition of enolase by fluoride leads to the accumulation of 2-phosphoglycerate, as this intermediate cannot proceed to the next step in glycolysis. Thus, the correct answer is A. 2-phosphoglycerate.
Q.10- A patient reports experiencing dizziness, fatigue, and tremors, and a fingerstick test reveals a blood glucose level of 45 mg/dl, indicating hypoglycemia. In response to such low glucose levels, glucagon is secreted. Which of the following allosteric modulators would be most effective in inhibiting glycolysis by not interacting with Phosphofructokinase-1 (PFK-1) under these circumstances?
A. ATP
B. Fr, 2,6 bisphosphate
C. Citrate
D. Glucose 6-P
E. NAD+
The correct answer is: B. Fructose 2,6-bisphosphate: Fructose 2,6-bisphosphate (F2,6BP) is a key activator of phosphofructokinase-1 (PFK-1), enhancing glycolysis. During hypoglycemia, glucagon is released, which decreases the levels of F2,6BP through the activation of fructose-2,6-bisphosphatase. This reduction in F2,6BP slows glycolysis indirectly, without direct interaction with PFK-1, because PFK-1’s activity depends on the presence of F2,6BP. Thus, the decrease in F2,6BP inhibits glycolysis without directly interacting with PFK-1.
Incorrect Options:
A. ATP: ATP inhibits glycolysis by directly interacting with PFK-1 as an allosteric inhibitor.
C. Citrate: Citrate also acts as an allosteric inhibitor by directly binding to PFK-1, signaling that energy is sufficient and slowing glycolysis.
D. Glucose 6-phosphate: G6P inhibits hexokinase, not PFK-1, but it does not have the same systemic role in the glucagon response as F2,6BP does in inhibiting glycolysis.
E. NAD⁺: NAD⁺ is a coenzyme required for glycolysis but is not a modulator involved in regulating glycolysis through glucagon or hypoglycemia.
Conclusion:
During hypoglycemia, the most effective way to inhibit glycolysis without directly interacting with PFK-1 is through lowering fructose 2,6-bisphosphate levels, which reduces PFK-1’s activity indirectly. Therefore, the correct answer is B. Fructose 2,6-bisphosphate.
Q.11- During her second trimester of pregnancy, a 30-year-old woman’s fetus efficiently acquires oxygen from maternal hemoglobin (HbA) due to a specific allosteric modifier that affects hemoglobin’s oxygen affinity. Which of the following compounds serves as this allosteric modifier, enhancing the oxygen-binding affinity of fetal hemoglobin (HbF) compared to maternal hemoglobin?
A. Acetyl CoA
B. 2,3 bisphosphoglycerate
C. Citrate
D. Fr,1,6 bisphosphate
E. Fr,2,6 bisphosphate
The correct answer is B. 2,3-bisphosphoglycerate (2,3-BPG): 2,3-bisphosphoglycerate (2,3-BPG) is a key allosteric modifier of hemoglobin that reduces hemoglobin’s affinity for oxygen by stabilizing the deoxygenated (T-state) form of hemoglobin. However, fetal hemoglobin (HbF) has a lower affinity for 2,3-BPG compared to maternal hemoglobin (HbA). This difference allows HbF to bind oxygen more tightly than HbA, facilitating the efficient transfer of oxygen from the mother’s blood to the fetus. During pregnancy, this mechanism is crucial because the fetus depends on the maternal oxygen supply for growth and development. By binding less 2,3-BPG, HbF remains in a high-affinity state for oxygen, giving it a competitive advantage in extracting oxygen from maternal hemoglobin.
Incorrect options:
A. Acetyl CoA:
Acetyl CoA is involved in energy metabolism and the TCA cycle but does not have any known role in modulating hemoglobin’s oxygen-binding affinity.
C. Citrate:
Citrate is a key intermediate in the TCA cycle and an allosteric regulator of enzymes in glycolysis, but it does not influence hemoglobin function.
D. Fructose 1,6-bisphosphate:
This glycolytic intermediate regulates glycolysis but has no effect on hemoglobin’s oxygen-binding properties.
E. Fructose 2,6-bisphosphate:
Fructose 2,6-bisphosphate is involved in glycolysis regulation, specifically in activating phosphofructokinase-1 (PFK-1). It does not interact with hemoglobin or affect oxygen binding.
Conclusion:
The most relevant allosteric modifier in this context is 2,3-bisphosphoglycerate (2,3-BPG). Its reduced binding to fetal hemoglobin (HbF) enhances the fetus’s ability to acquire oxygen from maternal hemoglobin (HbA), ensuring effective oxygen transfer during pregnancy. Therefore, the correct answer is B. 2,3-bisphosphoglycerate (2,3-BPG).
Q.12- Identify the enzyme with the correct modes of regulation
The correct answer is Pyruvate Kinase.
Based on the table, pyruvate kinase matches all the regulatory modes listed: allosteric modification, covalent modification, induction, and substrate concentration.
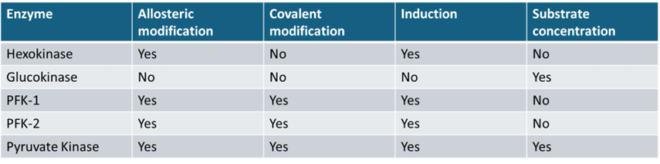
Explanation of Pyruvate Kinase Regulation:
1. Allosteric Modification:
Pyruvate kinase is activated by fructose-1,6-bisphosphate and inhibited by ATP and alanine, which signal high energy status.
2. Covalent Modification:
It is phosphorylated (inactivated) by glucagon during fasting, slowing glycolysis to conserve glucose.
3. Induction:
Its expression increases in response to insulin during fed states, promoting glycolysis.
4. Substrate Concentration:
The enzyme responds to levels of PEP (phosphoenolpyruvate), ensuring its activity aligns with the availability of substrates.
Conclusion:
Due to the presence of all these regulatory modes, Pyruvate Kinase is the correct answer.
Q.13- During intense muscular contraction, as compared to a resting state, which of the following metabolic changes is most likely observed in the muscle tissue?
A. Decreased concentration of AMP
B. Decreased level of fructose 2,6 bisphosphate
C. Decreased ratio of NADH/NAD+
D. Increased conversion of pyruvate to lactate
E. Increased oxidation of pyruvate to CO2
The correct answer is D. Increased conversion of pyruvate to lactate: During intense muscular contraction, oxygen delivery to the muscles becomes limited, and the energy demands of the muscle outpace the availability of oxygen. Under these anaerobic conditions, the muscle shifts toward anaerobic glycolysis, where pyruvate is converted to lactate by the enzyme lactate dehydrogenase. This conversion helps regenerate NAD⁺ from NADH, which is essential for maintaining glycolysis and ATP production to fuel muscle contraction.
Incorrect options
A (Decreased concentration of AMP) is incorrect because, during intense exercise, ATP is rapidly consumed, leading to an increase in AMP concentration. AMP serves as a signal to activate glycolysis and other pathways to meet energy demands.
B (Decreased level of fructose 2,6-bisphosphate) is incorrect because fructose 2,6-bisphosphate acts as a potent activator of phosphofructokinase-1 (PFK-1), promoting glycolysis. During exercise, its levels would increase to ensure glycolytic flux.
C (Decreased ratio of NADH/NAD⁺) is incorrect because anaerobic conditions promote the reduction of NAD⁺ to NADH, raising the NADH/NAD⁺ ratio. However, lactate formation helps regenerate NAD⁺ to maintain glycolysis.
E (Increased oxidation of pyruvate to CO₂) is incorrect because pyruvate oxidation to CO₂ via the TCA cycle and oxidative phosphorylation requires adequate oxygen. During intense contraction, oxygen becomes limiting, reducing the flux through the TCA cycle.
Thus, the increased conversion of pyruvate to lactate is the most likely metabolic change observed in muscle tissue during intense contraction.
Q.14- A teenager finishes eating a hot fudge sundae and a milkshake. As her blood sugar rises, the liver recruits both Glucokinase (km = 10.0m M) and Hexokinase (km = 0.10mM) to metabolize the glucose. Which of the following is a correct statement about the kinetics of these two enzymes?
A. Hexokinase will metabolize 100 times more glucose molecules
B. Hexokinase will lower the energy of activation more than glucokinase
C. Hexokinase has a much higher affinity to glucose than glucokinase
D. Hexokinase will have a higher Vmax than glucokinase
E. Hexokinase will be induced more than glucokinase by the rising concentration of glucose
The correct answer is C. Hexokinase has a much higher affinity to glucose than glucokinase. The Km value of an enzyme reflects its affinity for a substrate. A lower Km means the enzyme binds the substrate more tightly and reaches half of its maximum velocity (Vmax) at a lower concentration. In this case, hexokinase has a Km of 0.10 mM, meaning it has a much higher affinity for glucose than glucokinase, which has a Km of 10.0 mM. This indicates that hexokinase can efficiently phosphorylate glucose even at low glucose concentrations, whereas glucokinase only becomes more active at higher glucose concentrations, such as after a carbohydrate-rich meal.
Incorrect options:
A. Hexokinase will metabolize 100 times more glucose molecules:
This is incorrect because while hexokinase has a higher affinity for glucose, the actual amount of glucose metabolized (Vmax) depends on both the enzyme concentration and the substrate availability. Additionally, glucokinase in the liver is responsible for metabolizing large amounts of glucose when glucose levels are high, even though it has a higher Km.
B. Hexokinase will lower the energy of activation more than glucokinase:
Both hexokinase and glucokinase catalyze the same type of reaction (phosphorylation of glucose), so they reduce the activation energy similarly for this reaction.
D. Hexokinase will have a higher Vmax than glucokinase:
This is incorrect. Glucokinase has a higher Vmax than hexokinase, as it is adapted to handle large amounts of glucose that enter the liver after meals. Hexokinase, found in peripheral tissues, has a lower Vmax because it operates efficiently at lower glucose concentrations but saturates quickly.
E. Hexokinase will be induced more than glucokinase by the rising concentration of glucose:
This is incorrect because glucokinase is regulated by induction through insulin, particularly in the liver, following a rise in blood glucose. Hexokinase, on the other hand, is not inducible by glucose or insulin.
Q.15- Which of the following changes in enzyme activity will occur within 1 hour of a type 1 diabetic taking an injection of insulin?

The correct answer is A: Active kinase (PFK-2), Active kinase (PFK-1), Increased Muscle Glucose Uptake.
Explanation:
After an insulin injection, PFK-2 becomes active in its kinase form, increasing the production of fructose 2,6-bisphosphate, which activates PFK-1 and promotes glycolysis in the liver. Insulin also triggers the recruitment of GLUT-4 transporters to the muscle cell membrane, resulting in increased glucose uptake by muscle tissue. This combination ensures efficient glucose utilization and storage, which aligns with insulin’s metabolic effects.
Incorrect Options:
B: Inactive kinase (PFK-2), Active kinase (PFK-1), Increased Muscle Glucose Uptake
This is incorrect because insulin promotes the kinase activity of PFK-2, not its inactive form. The active kinase form of PFK-2 produces fructose 2,6-bisphosphate, which stimulates PFK-1 and promotes glycolysis. If PFK-2 were inactive, fructose 2,6-bisphosphate levels would drop, inhibiting PFK-1 activity and glycolysis. Thus, the inactive kinase form of PFK-2 does not align with insulin’s action.
C: Active phosphatase (PFK-2), Active kinase (PFK-1), Increased Muscle Glucose Uptake
This is incorrect because insulin inhibits the phosphatase activity of PFK-2. In its phosphatase form, PFK-2 decreases fructose 2,6-bisphosphate levels, reducing PFK-1 activity and slowing glycolysis. After insulin injection, PFK-2 remains in its active kinase form to enhance glycolysis, so this option is not correct.
D: Inactive phosphatase (PFK-2), Active kinase (PFK-1), Decreased Muscle Glucose Uptake
This option is incorrect because while insulin inhibits PFK-2’s phosphatase activity (as correctly stated), muscle glucose uptake increases, not decreases, following insulin injection. Insulin mobilizes GLUT-4 transporters to the muscle cell membrane, facilitating glucose entry into the cells, so a decrease in glucose uptake would not occur with insulin.
E: Active kinase (PFK-2), Inactive kinase (PFK-1), Decreased Muscle Glucose Uptake
This is incorrect because if PFK-1 were inactive, glycolysis would slow down or halt, which contradicts insulin’s effect of activating glycolysis in the liver. Additionally, muscle glucose uptake increases after insulin injection, making this option inconsistent with insulin’s physiological effects.
Conclusion:
The only correct option is A: Active kinase (PFK-2), Active kinase (PFK-1), Increased Muscle Glucose Uptake, which aligns with the metabolic actions of insulin in promoting glycolysis and glucose uptake.
Q.16- A 45-year-old woman is diagnosed with breast cancer. The oncologist orders a positron emission tomography(PET) scan of the head to rule out metastasis. This imaging modality covalently links a radioactive isotope, most commonly to Glucose, to appreciate highly active areas in the body such as tumors. Which of the following traps the tracer in the cell?
A. Insulin
B. GLUT-1
C. GLUT-4
D. Glucokinase
E. PFK-1
The correct answer is D. Glucokinase: In PET scans, the radioactive tracer is typically a glucose analog, such as fluorodeoxyglucose (FDG), which mimics glucose. After entering the cell, FDG undergoes phosphorylation by hexokinase (or glucokinase in the liver and pancreas) to form FDG-6-phosphate. Once phosphorylated, FDG-6-phosphate cannot proceed further through glycolysis, and it also cannot exit the cell because it is now trapped inside. This trapping allows PET imaging to highlight areas of high glucose metabolism, such as tumors or highly active tissues.
In the context of PET scans, GLUT-1 is indeed the transporter responsible for the uptake of glucose. After being transported into the cell, Glucose is then phosphorylated by hexokinase, which traps it inside the cell because G-6-phosphate cannot easily cross the cell membrane to exit the cell. While GLUT-1 is not directly responsible for the trapping mechanism—that is the job of hexokinase—it is responsible for the initial critical step of transporting the glucose analog into the cells. High GLUT-1 expression is often associated with cancer cells because they tend to have increased glucose uptake due to their high metabolic needs.
So in the process of PET scanning, GLUT-1 facilitates the entry of the radioactive tracer into the cells, where it gets trapped after phosphorylation. Therefore, the trapping of the tracer in the cell is a combined action of uptake and phosphorylation
Incorrect options:
A. Insulin: Insulin regulates glucose uptake, but it does not directly trap glucose or its analogs in the cell.
B. GLUT-1: GLUT-1 is a glucose transporter, facilitating glucose entry into cells, but it does not phosphorylate glucose.
C. GLUT-4: GLUT-4 is another glucose transporter, but it is primarily insulin-responsive and found in muscle and adipose tissue, not responsible for trapping glucose analogs.
E. PFK-1: Phosphofructokinase-1 (PFK-1) catalyzes a later step in glycolysis, not the initial trapping step.
Thus, the correct answer is glucokinase, which ensures that the radioactive glucose analog remains inside the cell for effective PET imaging.
Q.17- A 45-year-old obese man complains of having to get up frequently to urinate at night-time. He has also noticed that he is constantly thirsty and hungry. The patient is diagnosed with type 2 diabetes mellitus. If this is a problem at the level of glucose transporters, which tissue would be most affected?
A. Liver
B. Red blood cells
C. Muscle and adipose tissue
D. Brain tissue
E. Small intestine
The correct answer is C. Muscle and adipose tissue. In type 2 diabetes mellitus, insulin resistance impairs the function of GLUT-4, an insulin-dependent glucose transporter responsible for glucose uptake in muscle and fat cells. In healthy conditions, insulin promotes the translocation of GLUT-4 to the surface of these cells, allowing glucose entry. However, in insulin resistance, this process is disrupted, leading to reduced glucose uptake by these tissues and contributing to hyperglycemia, increased thirst, hunger, and frequent urination.
Incorrect options
The liver, which relies on GLUT-2 for glucose uptake, is not directly impacted by GLUT-4 dysfunction since GLUT-2 is insulin-independent. Similarly, red blood cells and brain tissue rely on GLUT-1 and GLUT-3, respectively, which do not require insulin for glucose transport. The small intestine uses sodium-glucose linked transporters (SGLTs) for absorption, which also function independently of insulin. Therefore, muscle and adipose tissues are most affected in type 2 diabetes due to their dependence on GLUT-4 for efficient glucose uptake.
Q.18- A 24-year-old woman is getting training for her first marathon. Her coach instructed her to keep a pace that allows her to stay below her anaerobic threshold. Under such conditions, pyruvate does not accumulate as it is converted to which of the following compounds?
A. Ethanol
B. Lactic acid
C. Acetyl CoA
D. Alanine
E. Oxaloacetate
The correct answer is C. Acetyl CoA: When a person exercises below their anaerobic threshold, oxygen supply is sufficient, and the muscles rely primarily on aerobic metabolism. Under these conditions, pyruvate is efficiently transported into the mitochondria, where it is converted into acetyl-CoA by the enzyme pyruvate dehydrogenase complex (PDC). Acetyl-CoA then enters the TCA cycle (Krebs cycle) to produce ATP through oxidative phosphorylation. As a result, pyruvate does not accumulate, and the energy demands are met efficiently through aerobic pathways.
Incorrect Options:
A: Ethanol – This option is incorrect because ethanol production occurs in microorganisms like yeast through alcoholic fermentation, not in human cells. Humans do not produce ethanol from pyruvate under physiological conditions.
B: Lactic acid – This option is incorrect in this context. Lactic acid is produced under anaerobic conditions, such as intense exercise when oxygen availability is limited, and pyruvate is converted to lactate to regenerate NAD⁺. Below the anaerobic threshold, aerobic metabolism predominates, and pyruvate is converted into acetyl-CoA instead of lactate.
D: Alanine – This is incorrect because pyruvate can be converted into alanine through transamination, but this pathway is not the primary route during exercise. Alanine plays a role in the glucose-alanine cycle during fasting or intense exercise, not under aerobic conditions typical during marathon training.
E: Oxaloacetate – This is incorrect because while pyruvate can be converted into oxaloacetate by pyruvate carboxylase, this reaction is mainly involved in gluconeogenesis or replenishing the TCA cycle intermediates (anaplerosis). During aerobic exercise, most pyruvate is directed toward acetyl-CoA production for energy.
Conclusion:
During marathon training below the anaerobic threshold, pyruvate is converted into acetyl-CoA, which fuels the TCA cycle and allows for efficient ATP production through aerobic metabolism. Thus, the correct answer is C: Acetyl CoA.
Q.19- Compared with the well-fed state, a fasting state shows which of the following?
A. A decreased concentration of glucagon
B. An increased conversion of pyruvate to lactate
C. Decreased level of Fr-2,6 bisphosphate
D. Increased level of Fr 1,6 bisphosphate
E. Increased concentration of Glucokinase
The correct answer is C: Decreased level of Fructose-2,6-bisphosphate: During fasting, the body shifts its metabolism to conserve glucose and mobilize stored energy. One major hormonal change is the increase in glucagon levels, which promotes gluconeogenesis and reduces glycolysis in the liver. Glucagon decreases the levels of fructose-2,6-bisphosphate (F2,6BP) by activating the phosphatase activity of PFK-2. Since F2,6BP is a potent activator of phosphofructokinase-1 (PFK-1), lowering its concentration decreases glycolysis and favors gluconeogenesis, ensuring the liver produces glucose for other tissues, such as the brain.
Incorrect Options:
A: A decreased concentration of glucagon – This is incorrect. During fasting, glucagon levels increase to promote gluconeogenesis and glycogenolysis, ensuring glucose availability for essential tissues.
B: An increased conversion of pyruvate to lactate – This is incorrect. While lactate production increases during anaerobic conditions (such as intense exercise), the liver focuses on gluconeogenesis during fasting, using pyruvate for glucose production rather than converting it to lactate.
D: Increased level of Fructose-1,6-bisphosphate – This is incorrect. Fructose-1,6-bisphosphate is an intermediate in glycolysis, and during fasting, glycolysis is downregulated in the liver. Instead, the focus is on gluconeogenesis, not glycolysis.
E: Increased concentration of Glucokinase – This is incorrect. Glucokinase is expressed in the liver and functions to phosphorylate glucose during fed states. Its expression is regulated by insulin, and during fasting, insulin levels are low, leading to a decrease in glucokinase activity.
Conclusion:
During fasting, fructose-2,6-bisphosphate levels decrease, reducing glycolysis and favoring gluconeogenesis to maintain blood glucose levels. Thus, the correct answer is C: Decreased level of Fructose-2,6-bisphosphate.
Q.20- A 24 -year-old man presented with symptoms of shortness of breath, weakness, and fatigue. His Hemoglobin level was 7G/dl. Red blood cells isolated from the patient showed abnormally low levels of lactate. A deficiency of which of the following enzymes would be the most likely cause for the patient’s anemia?
A. Glucokinase
B. Hexokinase
C. Phosphofructokinase
D. Phosphoglucose isomerase
E. Pyruvate kinase
The correct answer is E. Pyruvate kinase: Pyruvate kinase is a key enzyme in the last step of glycolysis, where it catalyzes the conversion of phosphoenolpyruvate (PEP) to pyruvate, generating ATP in the process. In red blood cells (RBCs), ATP production through glycolysis is essential because mature RBCs lack mitochondria and rely exclusively on glycolysis for energy. A deficiency in pyruvate kinase impairs glycolysis, leading to reduced levels of pyruvate and lactate.
This results in insufficient ATP production, which compromises the ability of the RBCs to maintain their membrane integrity and ion gradients. The RBCs become more prone to hemolysis, leading to hemolytic anemia. The patient’s symptoms of shortness of breath, weakness, and fatigue, along with a low hemoglobin level (7 g/dL) and low lactate levels, suggest a metabolic block in glycolysis at the pyruvate kinase step, impairing both energy production and lactate formation.
Incorrect Options:
A. Glucokinase – This enzyme phosphorylates glucose to glucose-6-phosphate in the liver and pancreas. A deficiency of glucokinase is associated with impaired glucose homeostasis (e.g., maturity-onset diabetes of the young) but does not affect RBC glycolysis, as RBCs do not express glucokinase.
B. Hexokinase – Hexokinase catalyzes the phosphorylation of glucose to glucose-6-phosphate in most tissues, including RBCs. While a hexokinase deficiency could impair glycolysis, it is very rare, and it would cause a generalized metabolic defect rather than specifically presenting with hemolytic anemia and low lactate.
C. Phosphofructokinase (PFK) – PFK is the rate-limiting enzyme in glycolysis. A deficiency in this enzyme can also impair glycolysis and lead to glycogen storage diseases (such as Tarui disease), but it is more commonly associated with exercise intolerance rather than isolated hemolytic anemia with low lactate levels.
D. Phosphoglucose isomerase – This enzyme catalyzes the conversion of glucose-6-phosphate to fructose-6-phosphate. Deficiency of this enzyme is rare but can also cause hemolytic anemia. However, it is less directly related to lactate production compared to pyruvate kinase deficiency, which impacts the final step of glycolysis.
Conclusion:
Given the combination of low hemoglobin levels (anemia) and abnormally low lactate levels, the most likely cause is a deficiency in pyruvate kinase. This condition directly affects glycolysis in RBCs, leading to hemolytic anemia. Therefore, the correct answer is E: Pyruvate kinase.
Q.21- After a heavy meal, which of the following allosteric activators would be most effective in increasing the rate of glycolysis?
A. ATP
B. Acetyl CoA
C. 2,3 bisphosphoglycerate
D. Citrate
E. Fr-2,6 bisphosphate
The correct answer is E. Fructose-2,6-bisphosphate: After a heavy meal, the body aims to utilize the incoming glucose by increasing glycolysis, particularly in the liver. Fructose-2,6-bisphosphate (F2,6BP) is a potent allosteric activator of phosphofructokinase-1 (PFK-1), the key regulatory enzyme of glycolysis. F2,6BP enhances the conversion of fructose-6-phosphate to fructose-1,6-bisphosphate, driving glycolysis forward. Insulin, which is released after a meal, promotes the kinase activity of PFK-2, increasing F2,6BP levels and stimulating glycolysis to efficiently process the elevated glucose levels.
Incorrect Options:
A. ATP – ATP acts as an allosteric inhibitor of PFK-1, signaling that the energy levels in the cell are sufficient. High ATP levels reduce the glycolytic rate, which is the opposite of what happens after a heavy meal.
B. Acetyl-CoA – Acetyl-CoA is not an activator of glycolysis. Instead, it inhibits pyruvate dehydrogenase and promotes gluconeogenesis, shifting metabolism away from glycolysis when energy levels are high or during fasting.
C. 2,3-bisphosphoglycerate (2,3-BPG) – 2,3-BPG plays a role in modulating oxygen affinity in hemoglobin but does not significantly affect the glycolytic pathway.
D. Citrate – Citrate is an allosteric inhibitor of PFK-1. High citrate levels indicate that the TCA cycle is well-supplied with intermediates, leading to a reduction in glycolysis.
Conclusion:
After a heavy meal, fructose-2,6-bisphosphate is the most effective allosteric activator to increase the rate of glycolysis by activating PFK-1. Thus, the correct answer is E: Fructose-2,6-bisphosphate.
Q.22-Match the columns

Correct Matching:
Ratios and effects are matched:

Explanation:
A: Increased NADH/NAD⁺ ratio → (Anaerobic glycolysis):
An increase in the NADH/NAD⁺ ratio is typically associated with anaerobic conditions, where pyruvate is converted to lactate to regenerate NAD⁺, enabling glycolysis to continue without oxygen.
B: Increased ATP/ADP ratio → (Energy rich state, decreased rate of glycolysis):
High ATP levels signal that the cell has sufficient energy, inhibiting glycolysis to prevent unnecessary glucose consumption.
C: Decreased ATP/AMP ratio → (Low energy state, increased rate of glycolysis):
A decrease in the ATP/AMP ratio indicates a low energy state, promoting glycolysis to rapidly produce ATP.
D: Increased Insulin/Glucagon ratio → (Well-fed state, increased rate of glycolysis):
After a meal, insulin levels rise, stimulating glycolysis to utilize the incoming glucose.
E: Increased Glucagon/Insulin ratio → (Fasting state, decreased rate of glycolysis):
During fasting, glucagon levels increase, promoting gluconeogenesis and suppressing glycolysis in the liver.
F: Increased NAD⁺/NADH ratio → (Continuation of glycolysis):
A higher NAD⁺ concentration promotes glycolysis by maintaining the necessary redox balance for glycolytic reactions to proceed efficiently.
This corrected matching accurately reflects the physiological effects of the given ratios.
Q.23- Identify the correct effect of allosteric effectors on the given enzymes:
The correct combinations are as shown in the image below:
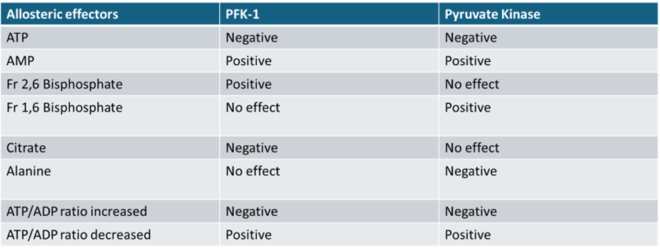
PFK-1:
o Negative: ATP, Citrate, Increased ATP/ADP Ratio
o Positive: AMP, Fructose-2,6-bisphosphate, Decreased ATP/ADP Ratio
Pyruvate Kinase:
o Negative: ATP, Alanine, Increased ATP/ADP Ratio
o Positive: AMP, Fructose-1,6-bisphosphate, Decreased ATP/ADP Ratio
