Library
Hypoglycemia Due To Biotin deficiency
- February 22, 2020
- Posted by: Namrata Chhabra
- Category: Energy metabolism Metabolism of Carbohydrates Multiple-Choice questions Multiple-choice questions Multiple-choice questions Multiple-Choice questions Practice questions USMLE Style questions USMLE styled question bank Vitamins Vitamins and Minerals
A 32-year-old bodybuilder has decided to go on a diet consisting of egg whites to ensure only proteins for muscle growth. After a few weeks, he experiences decreased energy and is found to be hypoglycemic. A nutritionist tells the patient that he most likely has a deficiency of vitamin Biotin. Which of the following enzymes cannot catalyze its step in synthesizing glucose from pyruvate?
A) Pyruvate carboxylase
B) Phosphoenol pyruvate carboxykinase
C) Glucose-6-phosphatase
D) Fructose 1, 6 bisphosphatase
E) Phosphoglycerate kinase.
The correct answer is- A) – Pyruvate carboxylase.
Biotin deficiency is very common in bodybuilders who consume raw egg whites. The Raw egg whites contain Avidin, a glycoprotein that strongly binds with biotin and prevents its absorption. Once a biotin-Avidin complex forms, the bond is essentially irreversible; the biotin-Avidin complex is not broken during the passage of the food bolus through the stomach and intestines. As a result, biotin is not liberated from food, and the biotin-Avidin complex is lost in the feces. Thus, ingesting large quantities of raw egg whites over a long period can result in a biotin deficiency. Cooking egg white denatures Avidin, rendering it susceptible to digestion and, therefore, unable to prevent the absorption of dietary biotin.
Biotin functions to transfer carbon dioxide in a small number of carboxylation reactions. Biotin is attached to the active site of carboxylases.
Each biotin-dependent carboxylase catalyzes an essential metabolic reaction:
1) Pyruvate carboxylase
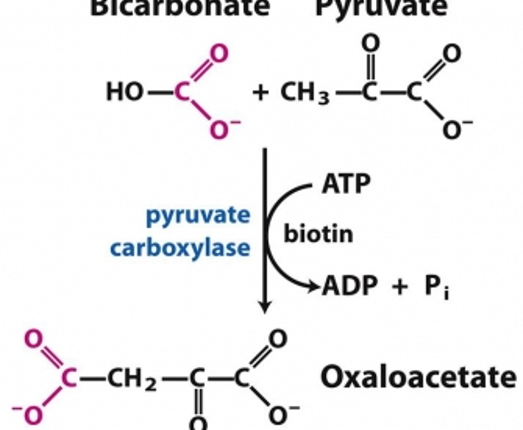
Pyruvate carboxylase is a critical enzyme in gluconeogenesis—the formation of glucose from sources other than carbohydrates, for example, amino acids. Oxaloacetate formed from pyruvate can be utilized in many other ways depending upon the needs of the cell (Figure 3)
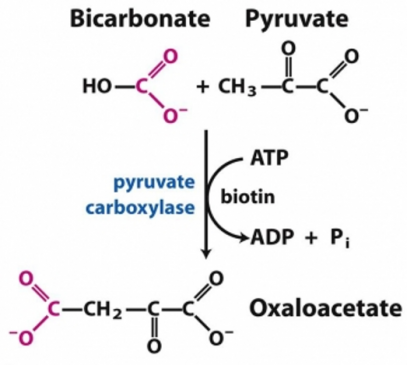
Figure 1 -Carboxylation of pyruvate to Oxaloacetate, catalyzed by Pyruvate carboxylase, is the first step of gluconeogenesis.
The other examples where Biotin is required as a coenzyme are:
2) Acetyl-CoA carboxylase (ACC) catalyzes the binding of bicarbonate to acetyl-CoA to form malonyl-CoA (Figure-2). Malonyl-CoA is required for the synthesis of fatty acids.
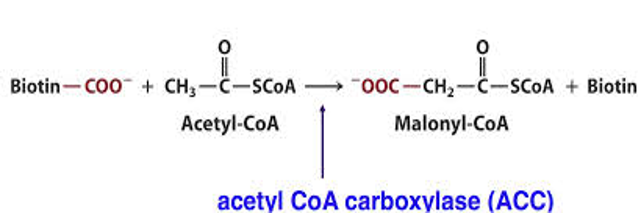
Figure 2 -The carboxylation of Acetyl CoA to form Malonyl CoA, catalyzed by Acetyl CoA carboxylase, is the first and the rate-limiting step in fatty acid synthesis.
3) Propionyl-CoA carboxylase catalyzes essential steps in the metabolism of certain amino acids, cholesterol, and odd-chain fatty acids (fatty acids with an odd number of carbon molecules).
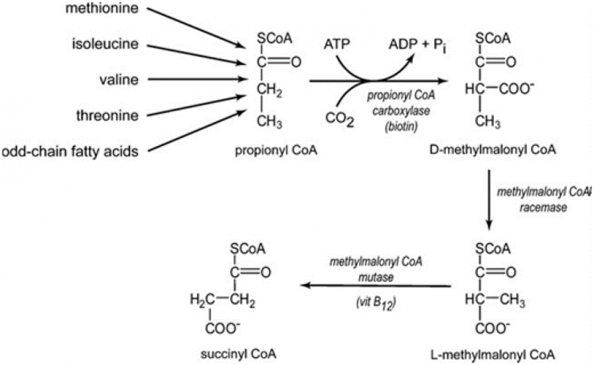
Figure- 3-showing the fate of Propionyl CoA.
Propionyl CoA is converted first to D- Methyl malonyl CoA and then to its L isomer, ultimately to succinyl CoA for complete utilization in the TCA cycle (figure 3).
Anaplerotic reactions catalyzed by biotin-dependent pyruvate carboxylase (PC) and Propionyl-coenzyme A carboxylase (PCC) regenerate oxaloacetate for the citric acid cycle.
As regards other options:
B) Phosphoenol pyruvate carboxykinase– Catalyzes the conversion of Oxaloacetate to phosphoenolpyruvate, it is an enzyme of a pathway of gluconeogenesis, but it is not a biotin-dependent enzyme
C) Glucose-6-phosphatase catalyzes the last step of converting glucose 6-phosphate to free glucose, but it is also not a biotin-dependent enzyme.
D) Fructose 1, 6 bisphosphatase– catalyzes the conversion of fructose1, 6-bisphosphates to Fructose 6 Phosphate; it is also not a biotin-dependent enzyme.
E) Phosphoglycerate kinase- is a common enzyme, both for the pathway of glycolysis and gluconeogenesis. It catalyzes the reversible conversion of 1, 3 bisphosphoglycerate to 3, phosphoglycerate; ATP is formed by substrate-level phosphorylation in glycolysis. ATP is consumed in the reverse reaction in the pathway of gluconeogenesis.
Author:Namrata Chhabra
Leave a Reply Cancel reply
You must be logged in to post a comment.
