Library
Solved questions on Polysaccharides
- November 9, 2019
- Posted by: Namrata Chhabra
- Category: Learning resources Chemistry of Carbohydrates Library Short-answer questions
Q.1- What are storage polysaccharides? Give a brief description of each of them.
Answer- Glycogen, starch, and Inulin are storage polysaccharides.
1) Glycogen- Glycogen is a readily mobilized storage form of glucose. It is a very large, branched polymer of glucose residues (Figure-1) that can be broken down to yield glucose molecules when energy is needed. Most of the glucose residues in glycogen are linked by α-1,4-glycosidic bonds. Branches at about every tenth residue are created by α-1,6-glycosidic bonds. It is the storage polysaccharide in animals and is sometimes called animal starch, but it is more branched than amylopectin present in starch.
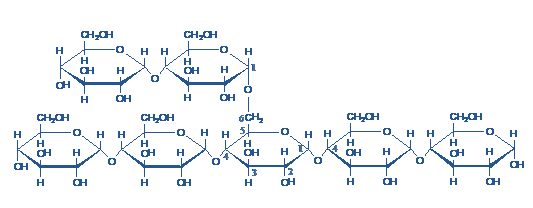
Figure- 1-showing the structure of glycogen, Note the α-1,6-glycosidic linkage at the branch point
It is hydrolyzed by both α and β-amylases and by glycogen phosphorylase. The complete hydrolysis yields glucose. Glycogen on reaction with iodine gives a reddish-brown color.
Glycogen is stored in muscle and liver. The concentration of glycogen is higher in the liver than in muscle (10%versus 2% by weight), but more glycogen is stored in skeletal muscle overall because of its much greater mass. Glycogen is present in the cytosol in the form of granules ranging in diameter from 10 to 40 nm. In the liver, glycogen synthesis and degradation are regulated to maintain blood glucose levels as required to meet the needs of the organism as a whole. In contrast, in muscle, these processes are regulated to meet the energy needs of the muscle itself.
Glycogen is not as reduced as fatty acids are and consequently not as energy-rich. But still, animals store energy as glycogen? All excess fuel is not converted to fatty acids. Glycogen is an important fuel reserve for several reasons.
The controlled breakdown of glycogen and the release of glucose increase the amount of glucose that is available between meals. Hence, glycogen serves as a buffer to maintain blood glucose levels. Glycogen’s role in maintaining blood glucose levels is especially important because glucose is virtually the only fuel used by the brain, except during prolonged starvation. Moreover, the glucose from glycogen is readily mobilized and is, therefore, a good source of energy for sudden, strenuous activity. Unlike fatty acids, the released glucose can provide energy in the absence of oxygen and can thus supply energy for anaerobic activity.
2) Starch- It is a polymer of glucose, found in roots, rhizomes, seeds, stems, tubers, and corms of plants, as microscopic granules having characteristic shapes and sizes. Most animals, including humans, depend on these plant starches for nourishment. The intact granules are insoluble in cold water, but grinding or swelling them in warm water causes them to burst. The released starch consists of two fractions.
About 20% is a water-soluble material called Amylose. The majority of the starch is a much higher molecular weight substance, consisting of nearly a million glucose units, and called amylopectin.
(a) Amylose is a linear polymer of α-D-glucose, linked together by α 1→4 glycosidic linkages. It is soluble in water, reacts with iodine to give a blue color and the molecular weight of Amylose ranges between 50, 000 – 200, 000.
(b) Amylopectin is a highly branched polymer, insoluble in water, reacts with iodine to give a reddish violet color. The molecular weight ranges between 70, 000 – 1 000, 000. Branches are composed of 25-30 glucose units linked by α 1→4 glycosidic linkage in the chain and by α 1→6 glycosidic linkage at the branch point.
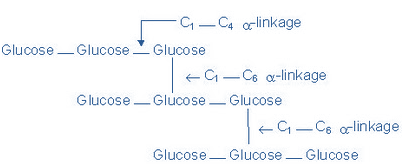
Figure-2 showing the structure of Amylopectin
Hydrolysis: Hydrolysis of starch with hot dilute acids or by enzymes gives dextrins of varying complexities, maltose and finally D-glucose
3) Inulin- Inulin is a polysaccharide of fructose (and hence a fructosan) found in tubers and roots of dahlias, artichokes,
and dandelions. It is readily soluble in water and is not hydrolyzed by intestinal enzymes. It has a lower molecular weight than starch and colors yellow with iodine.
It is used to determine the glomerular filtration rate, Inulin is of particular use as it is not secreted or reabsorbed in any appreciable amount at the nephron allowing GFR to be calculated, rather than total renal filtration. However, due to clinical limitations, Inulin is rarely used for this purpose and creatinine values are the standard for determining an approximate GFR.
Q.2- What are structural polysaccharides? Give a brief account of each of them.
Answer- Cellulose and chitin are structural polysaccharides.
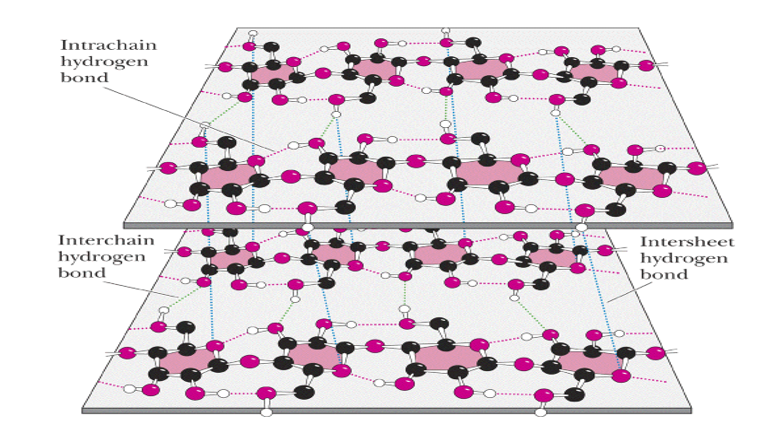
1) Cellulose-Cellulose is the chief constituent of plant cell walls. It is the most abundant of all carbohydrates. It is insoluble in water, gives no color with iodine and consists of β -D-glucopyranose units linked byβ 1 →4 bonds to form long, straight chains strengthened by cross-linking hydrogen bonds. Mammals lack an enzyme that hydrolyzes the β 1→ 4 bonds, and so cannot digest cellulose. It is an important source of “bulk” in the diet and the major component of dietary fiber. Microorganisms in the gut of ruminants and other herbivores can hydrolyze the linkage and ferment the products to short-chain fatty acids as a major energy source. There is some bacterial metabolism of cellulose in the human colon.
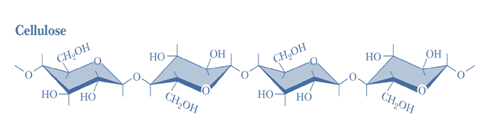
Figure-3- showing the structure of cellulose
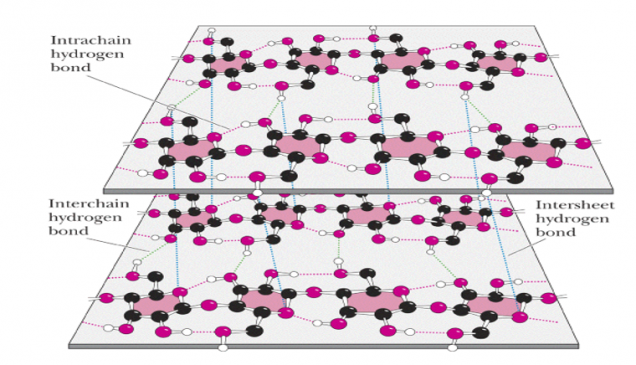

Figure-4- Showing the intrachain and interchain hydrogen bonding in the cellulose molecule
Cellulose yields Glucose upon complete hydrolysis. Partial hydrolysis yields cellobiose.
Products obtained from Cellulose-
• Microcrystalline cellulose: used as binder-disintegrant in tablets
• Methylcellulose: suspending agent and bulk laxative
• Oxidized cellulose: hemostat
• Sodium carboxymethyl cellulose: laxative
• Cellulose acetate:rayon; photographic film; plastics
• Cellulose acetate phthalate: enteric coating
• Nitrocellulose:explosives; collodion (pyroxylin)
2) Chitin is a structural polysaccharide in the exoskeleton of crustaceans and insects, and also in mushrooms. It consists of N-acetyl-D-Glucosamine units joined by β 1→ 4 glycosidic bonds. Chitin is the second most abundant carbohydrate polymer and is used commercially in coatings (extends the shelf life of fruits and meats).
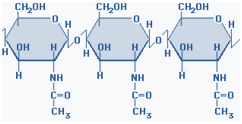
Figure-5- showing the structure of chitin
Q.3 – What is the difference between dextrins and dextrans?
Answer-
Dextrins- are
•produced along with maltose and glucose by the partial hydrolysis of starch
•dextrins are often referred to as either Amylo dextrins, erythrodextrins or achrodextrins
•used as mucilages (glues)
•also used in infant formulas(prevent the curdling of milk in baby’s stomach)
Dextrans- are
•products of the reaction of glucose and the enzyme Transglucosidase from Leuconostoc mesenteroides
•contains α (1,4), α (1,6) and α (1,3) linkages
•MW: 40,000; 70,000; 75,000
•used as plasma expanders(treatment of shock)
•also used as molecular sieves to separate proteins and other large molecules (gel filtration chromatography)
•Components of dental plaques.
Q.4- What are Glycosaminoglycans? Discuss the structure and functions of various Glycosaminoglycans.
Answer- The most abundant heteropolysaccharides in the body are the glycosaminoglycans (GAGs). GAGs are highly negatively charged molecules, with extended conformation that imparts high viscosity to the solution. GAGs are located primarily on the surface of cells or in the extracellular matrix (ECM). Along with the high viscosity of GAGs comes low compressibility, which makes these molecules ideal for a lubricating fluid in the joints. At the same time, their rigidity provides structural integrity to cells and provides passageways between cells, allowing for cell migration.
The specific GAGs of physiological significance are hyaluronic acid, dermatan sulfate, Chondroitin sulfate, heparin, heparan sulfate, and keratan sulfate. These molecules are long unbranched polysaccharides containing a repeating disaccharide unit. [acidic sugar-amino sugar]n
Although each of these GAGs has a predominant disaccharide component, heterogeneity does exist in the sugars present in the make-up of any given class of GAG. The disaccharide units contain either of two modified sugars, N-acetyl galactosamine (GalNAc) or N-acetylglucosamine (GlcNAc), as amino sugars and uronic acid such as glucuronate or Iduronate as acidic sugars.
The amino sugar may also be sulfated on carbon 4 or 6 or on non-acetylated nitrogen. The acidic sugars contain carboxyl groups that are negatively charged at physiological pH, and together with the sulfate groups, give glycosaminoglycans their strongly negative nature.
Because of their large number of negative charges, these heteropolysaccharide chains tend to be extended in solution. They repel each other and are surrounded by a shell of water molecules. When brought together they “slip” past each other. This produces the slippery consistency of mucous secretions and synovial fluid. When a solution of GAG is compressed, the water is squeezed out and GAGs are forced to occupy a smaller volume. When the compression is released the GAGs get back to their original, hydrated volume because of the repulsion of the negative charges. This property contributes to the resilience of synovial fluid and vitreous humor of eye.
THE SPECIFIC GAGs OF PHYSIOLOGICAL SIGNIFICANCE ARE:
1) Hyaluronic acid – The repeating disaccharide unit is N-Acetylglucosamineand Glucuronic acid.
(D-glucuronate + GlcNAc) n
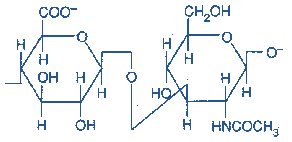
Figure-6-showing the structure of Hyaluronic acid
Occurrence: Hyaluronic acid is found in synovial fluid, ECM of loose connective tissue, umbilical cord and vitreous humor of the eye. It serves as a lubricant and shock absorber. It is the only GAG that is not limited to animal tissue but is also found in bacteria.
Hyaluronic acid is unique among the GAGs because it does not contain any sulfate and is not found covalently attached to proteins. It forms non-covalently linked complexes with Proteoglycans in the ECM.
Hyaluronic acid polymers are very large (100 – 10,000 kDa) and can displace a large volume of water.
2) Dermatan sulfate- The repeating disaccharide unit is N-Acetyl Galactosamineand L-Iduronic acid, with a variable amount of Glucuronic acids.
(L-Iduronate + GalNAc sulfate) n

Figure-7-showing the structure of Dermatan Sulfate
Occurrence: It is found in skin, blood vessels, and heart valves
3) Chondroitin sulfate- The repeating disaccharide unit is N-Acetyl galactosamine with sulfate on either C-4 or C-6 and Glucuronic acid. Based on the presence of the sulfate group, it may be labeled as Chondroitin-4-Sulfate or Chondroitin-6-Sulfate.
(D-glucuronate + GalNAc sulfate)n
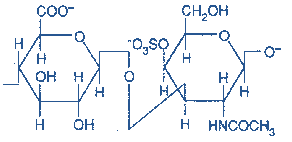
Figure-8-showing the structure of Chondroitin Sulfate
Occurrence: It is found in cartilages, tendons, ligaments, heart valves and aorta.
It is the most abundant GAG. In cartilages, they bind collagen and hold fibers in a tight, strong network.
4) Heparin sulfate – The repeating disaccharide units
D- Glucosamine and L-Iduronic acid with variable amounts of Glucuronic acid. Most glucosamine residues are bound in Sulfamide linkages. Sulfate is also found on C-3 or C-6 of Glucosamine and C-2 of uronic acid (An average of 2.5 Sulfate per disaccharide unit)
(D-glucuronate sulfate +N-sulfo-D-glucosamine) n
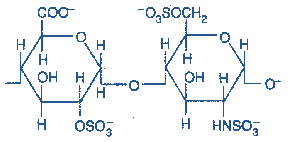
Figure-9-Showing the structure of Heparin Sulfate
Occurrence: Heparin is a component of intracellular granules of mast cells lining the arteries of the lungs, liver, and skin (Contrary to other GAGs that are extracellular compounds, it is intracellular). It serves as an anticoagulant.
5) Heparan sulfate: Heparans have fewer sulfate groups than heparins. The repeating disaccharide unit is the same as Heparin. Some glucosamines are acetylated
Occurrence- It is an extracellular GAG found in the basement membrane and as a ubiquitous component of cell surfaces
6) Keratan sulfate –The repeating disaccharide units N-Acetyl glucosamine and galactose (No uronic acid). The sulfate content is variable and may be present on C-6 of either sugar.
(Gal + GlcNAc sulfate) n
Most heterogeneous GAGs because they contain additional monosaccharides such as L-fucose, N-Acetyl Neuraminic acid, and Mannose.
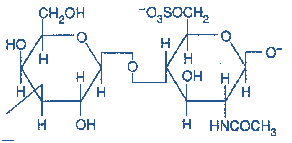
Figure-10- showing the structure of Keratan sulfate
Occurrence: cornea,bone, cartilage;
Keratan sulfates are often aggregated with Chondroitin sulfates.
Proteoglycans (mucoproteins) are formed of glycosaminoglycans (GAGs) covalently attached to the core proteins. They are found in all connective tissues, extracellular matrix (ECM) and on the surfaces of many cell types. Proteoglycans are remarkable for their diversity (different cores, different numbers of GAGs with various lengths and compositions).
Structure of Proteoglycans
All of the GAGs, except Hyaluronic acid, are found covalently attached to protein forming proteoglycan monomers.
Structure of Proteoglycan monomer
A Proteoglycan monomer found in cartilage consists of a core protein to which the linear GAG chains are covalently linked. These chains which each may be composed of more than 100 monosaccharides, extend out from the core protein and remain separated from each other because of charge repulsion. The resulting structure resembles a ‘Bottlebrush’(see figure). In cartilage proteoglycans, the species of glycosaminoglycans include Chondroitin sulfate and Keratan sulfate.
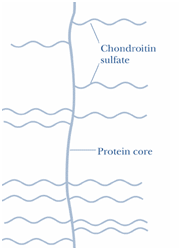
Figure- 11-showing the structure of Proteoglycan monomer(Bottle Brush)
The Linkage between the carbohydrate chain and the protein
The linkage of GAGs such as (heparan sulfates and Chondroitin sulfates) to the protein core involves a specific trisaccharide linker (Galactose-galactose-Xylose). The protein cores of Proteoglycans are rich in Ser and Threonine residues which allow multiple GAG attachments.
An O-Glycosidic bond is formed between the Xylose and the hydroxyl group of Serine. Some forms of keratan sulfates are linked to the protein core through an
N-asparaginyl bond (N-glycosidic linkage )
Proteoglycan Aggregates- The proteoglycan monomers associate with a molecule of Hyaluronic acid to form Proteoglycan aggregates. The association is not covalent but occurs primarily through ionic interactions between the core protein and Hyaluronic acid. The association is stabilized by additional small proteins called Link proteins.
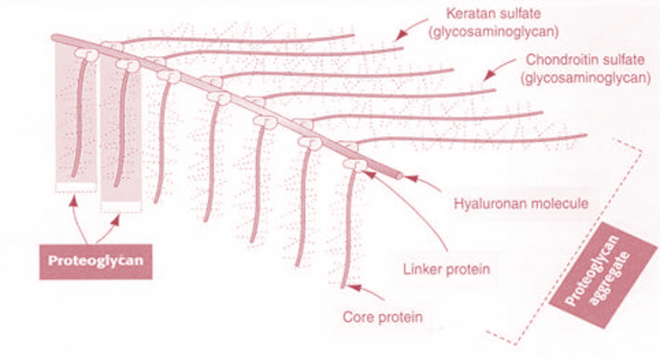

Figure-12- showing the structure of proteoglycan aggregate
Functions of Proteoglycans
They perform numerous vital functions within the body.
GAG dependent functions can be divided into two classes: the biophysical and the biochemical.
1) The biophysical functions depend on the unique properties of GAGs: the ability to fill the space, bind and organize water molecules and repel negatively charged molecules. Because of high viscosity and low compressibility they are ideal for a lubricating fluid in the joints. On the other hand, their rigidity provides structural integrity to the cells and allows cell migration due to providing the passageways between cells.
2) The other, more biochemical functions of GAGs are mediated by specific binding of GAGs to other macromolecules, mostly proteins. Proteoglycansparticipate in cell and tissue development and physiology.
3) Heparin acts as an anticoagulant and is used in clinical practice.
Q.5-What do you understand by the term mucopolysaccharidoses? Give a brief account in a tabular manner.
Answer-Glycosaminoglycans are degraded by Lysosomal Hydrolases. A deficiency of one of the Hydrolase results in mucopolysaccharidoses. These are hereditary disorders in which the Glycosaminoglycans accumulate in tissues, causing symptoms such as skeletal and extracellular matrix deformities, and mental retardation.
| Type | Main diseases | Deficient enzyme | Accumulated products | Symptoms |
| MPS I | Hurler’s syndrome | α-L-Iduronidase |
Heparan sulfate Dermatan sulfate |
Mental retardation Micrognathia Coarse facies Macroglossia Retinal degeneration Corneal clouding Cardiomyopathy |
| MPS II | Hunter syndrome | Iduronate sulfatase |
Heparan sulfate Dermatan sulfate |
Mental retardation(similar, but milder, symptoms to Hurler syndrome) |
| MPS III | Sanfilippo syndrome A | Heparan sulfamidase | Heparan sulfate |
Developmental delay Severe hyperactivity Spasticity Motor dysfunction death by the second decade |
| Sanfilippo syndrome B | N-acetylglucosaminidase | |||
| Sanfilippo syndrome C | Acetyl-CoA:alpha-glucosaminide acetyltransferase | |||
| Sanfilippo syndrome D | N-acetyl glucosamine 6-sulfatase | |||
| MPS IV | Morquio syndrome A | Galactose-6-sulfate sulfatase |
Keratan sulfate Chondroitin 6-sulfate |
Severe skeletal dysplasia Short stature Motor dysfunction |
| Morquio syndrome B | Beta-galactosidase | Keratan sulfate | ||
| MPS VI | Maroteaux-Lamy syndrome | N-acetylgalactosamine-4-sulfatase | Dermatan sulfate |
Severe skeletal dysplasia Short stature Motor dysfunction KyphosisHeart defects |
| MPS VII | Sly syndrome | β-glucuronidase |
Heparan sulfate Dermatan sulfate Chondroitin 4,6-sulfate |
Hepatomegaly Skeletal dysplasia Short stature Corneal clouding Developmental delay |
| MPS IX | Natowicz syndrome | Hyaluronidase | Hyaluronic acid |
Nodular soft-tissue masses around joint episodes of painful swelling of the masses Short-term pain Mild facial changes short stature Normal joint movement Normal intelligence |
Laboratory Investigations
1) Urine test, which shows the excessive excretion of undegraded mucopolysaccharides, which is specific for a specific type.
2) Cetyl Trimethyl ammonium bromide test is undertaken to confirm the presence of glycosaminoglycans in the urine.
3) Absence of Lysosomal enzyme in cultured fibroblasts.
4) Culture of cells from amniotic fluid obtained by amniocentesis for enzyme testing (prenatal testing)
5) X-ray of the spine and chest.
Prenatal diagnosis using amniocentesis and chorionic villus sampling can verify if a fetus either carries a copy of the defective gene or is affected with the disorder. Genetic counseling can help parents who have a family history of the Mucopolysaccharidoses determine if they are carrying the mutated gene that causes the disorders.
Treatment
This disease can be treated by bone marrow transplantation (BMT) and umbilical cord blood transplantation (UCBT) preferably before the age of 18 months. Abnormal physical characteristics, except for those affecting the skeleton and eyes, can be improved, and neurologic degeneration can often be halted. BMT and UCBT are high-risk procedures with high rates of morbidity and mortality. There is no cure for Mucopolysaccharidoses.
Gene therapy is under trial as a permanent cure. Enzyme replacement therapies are currently in use, they have proven useful in reducing non-neurological symptoms and pain.
Author:Namrata Chhabra
Leave a Reply Cancel reply
You must be logged in to post a comment.
