Library
Short-answers reasoning questions on Fatty acids and Triglycerides synthesis
- November 12, 2024
- Posted by: Namrata Chhabra
- Category: Energy metabolism Learning resources Library Metabolism of lipids Quick Revision Series Short-answer questions Short-Answer questions USMLE Content
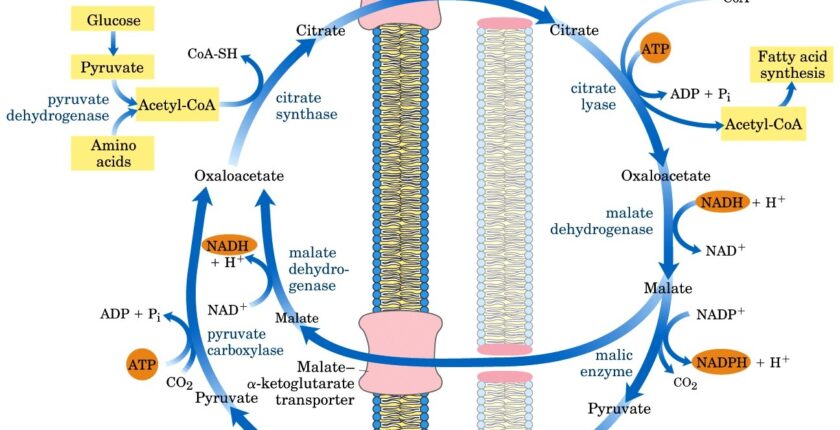
Question 1: Where does fatty acid synthesis primarily occur within the cell?
Answer: Fatty acid synthesis occurs in the cytosol of the cell. This is in contrast to fatty acid oxidation, which primarily occurs in the mitochondria.
Question 2: What is the primary product of fatty acid synthesis?
Answer: The primary product of fatty acid synthesis is palmitate, a 16-carbon saturated fatty acid. Palmitate can then be further modified by elongation and desaturation processes to create a variety of other fatty acids.
Question 3: Name two sources of NADPH required for fatty acid synthesis.
Answer: Fatty acid synthesis requires reducing power in the form of NADPH. The sources are:
○ The oxidative reactions of the pentose phosphate pathway are the primary source of NADPH for fatty acid synthesis. This pathway is particularly active in tissues specializing in lipogenesis, like the liver, adipose tissue, and lactating mammary gland.
○ The conversion of malate to pyruvate, catalyzed by malic enzyme (NADP-dependent malate dehydrogenase), also generates NADPH. While this may not be the main source in all tissues, it contributes to the NADPH pool needed for fatty acid synthesis.
Question 4: What molecule is responsible for transporting acetyl CoA from the mitochondria to the cytosol for fatty acid synthesis?
Answer: Citrate is responsible for transporting acetyl CoA from the mitochondria to the cytosol. Acetyl CoA, generated within the mitochondria, cannot directly cross the mitochondrial membrane. Instead, it is converted to citrate within the mitochondria, transported to the cytosol, and then cleaved back into acetyl CoA and oxaloacetate by the enzyme citrate lyase.
Question 5: What is the role of citrate in fatty acid synthesis regulation?
Answer: Citrate plays a crucial role in fatty acid synthesis regulation in two main ways:
○ Allosteric activation of acetyl CoA carboxylase: Citrate acts as an allosteric activator of acetyl CoA carboxylase, the enzyme that catalyzes the first committed step in fatty acid synthesis. This activation occurs when citrate levels are high, indicating a plentiful supply of acetyl CoA and energy for fatty acid synthesis.
○ Precursor for cytosolic acetyl CoA: As described in the previous answer, citrate is transported from the mitochondria to the cytosol, where it is cleaved to release acetyl CoA, the building block for fatty acid synthesis. Thus, citrate serves as both a signal for fatty acid synthesis and a source of the primary substrate.
Question 6: How do long-chain acyl CoA molecules regulate acetyl CoA carboxylase activity?
Answer: Long-chain acyl CoA molecules act as feedback inhibitors of acetyl CoA carboxylase. This means that when levels of long-chain fatty acids (and their corresponding acyl CoA derivatives) are high, they inhibit the enzyme responsible for initiating fatty acid synthesis, preventing the overproduction of fatty acids.
Question 7: What is the effect of insulin on fatty acid synthesis?
Answer: Insulin stimulates fatty acid synthesis. It achieves this through several mechanisms:
○ Covalent modification: Insulin promotes the dephosphorylation and activation of acetyl CoA carboxylase, increasing its activity.
○ Induction of enzyme synthesis: Insulin stimulates the gene expression of enzymes involved in fatty acid synthesis, such as acetyl CoA carboxylase and fatty acid synthase, leading to increased enzyme levels and enhanced fatty acid synthesis capacity.
○ Glycerol-3-phosphate provision: Insulin enhances glucose uptake and utilization through the glycolytic pathway, leading to an increase in the production of glycerol-3-phosphate, which is essential for the esterification of fatty acids into triacylglycerols (TAGs).
Question 8: How does malonyl CoA regulate beta-oxidation of fatty acids?
Answer: Malonyl CoA inhibits the enzyme carnitine palmitoyltransferase I (CPT I). CPT I is responsible for transporting fatty acids into the mitochondria for beta-oxidation. Thus, when malonyl CoA levels are high (as in the well-fed state when fatty acid synthesis is active), the breakdown of newly synthesized fatty acids is prevented by inhibiting their entry into the mitochondria.
Question 9: What are the two most common monounsaturated fatty acids produced from palmitate and stearate?
Answer: The two most common monounsaturated fatty acids produced from palmitate and stearate are:
○ Palmitoleate (16:1 Δ9): Formed by the desaturation of palmitate.
○ Oleate (18:1 Δ9): Formed by the desaturation of stearate.
Question 10: Why are linoleic acid and linolenic acid considered essential fatty acids?
Answer: Linoleic acid and linolenic acid are considered essential fatty acids because humans lack the enzymes necessary to introduce double bonds beyond the Δ9 position in fatty acids. This means we cannot synthesize these polyunsaturated fatty acids and must obtain them from our diet. These fatty acids are precursors for other important molecules, including arachidonic acid, and play vital roles in various physiological processes.
Question 11: What are the primary tissues or organs involved in fatty acid synthesis?
Answer: The fatty acid synthesis occurs in a variety of tissues, including the liver, kidney, brain, lung, mammary gland, and adipose tissue. However, the major sites for TAG synthesis are the liver, adipose tissues, and lactating mammary glands.
Question 12: Explain the role of acetyl CoA carboxylase in fatty acid synthesis.
Answer: This irreversible reaction is a key regulatory point in the pathway, and the enzyme itself is subject to various forms of regulation, including allosteric control, feedback inhibition, covalent modification, and conformational changes.
Question 13: Describe the structure of the fatty acid synthase complex and its role in fatty acid synthesis.
Answer: The fatty acid synthase complex is a dimer composed of two identical monomers. Notably, each monomer contains all seven enzyme activities required for fatty acid synthesis on a single polypeptide chain. This unique structure ensures coordinated synthesis and efficient channeling of intermediates during the cyclical process of fatty acid synthesis.
Question 14: Outline the steps involved in one cycle of fatty acid synthesis after the initial activation of acetyl and malonyl groups.
Answer: After the activation of acetyl and malonyl groups onto the fatty acid synthase complex, each cycle of fatty acid synthesis involves four key steps:
- Condensation: An acetyl group and a malonyl group condense, releasing carbon dioxide and forming a four-carbon unit.
- Reduction: The carbonyl group of the four-carbon unit is reduced to a hydroxyl group using NADPH.
- Dehydration: A water molecule is removed, forming a double bond.
- Reduction: The double bond is reduced to a single bond using another molecule of NADPH.
These steps are repeated, with the growing fatty acid chain extended by two carbons in each cycle, until palmitate (16 carbons) is synthesized.
Question 15: What is the final product released from the fatty acid synthase complex, and how is it released?
Answer: The final product released from the fatty acid synthase complex is free palmitate. After seven cycles of condensation and reduction, the 16-carbon saturated palmitoyl group, still attached to the acyl carrier protein (ACP), is released by hydrolytic activity within the synthase complex.
Question 16: How do dietary conditions (high-carbohydrate diet vs. low-carbohydrate/low-calorie diet) influence the long-term regulation of acetyl CoA carboxylase?
Answer: High-carbohydrate diet: A prolonged high-calorie or high-carbohydrate diet leads to an increase in acetyl CoA carboxylase concentration by enhancing gene expression (induction) of the enzyme and fatty acid synthase. This upregulation reflects the abundance of substrate and energy available for fatty acid synthesis.
○ Low-carbohydrate/low-calorie diet: A low-calorie diet or fasting results in decreased fatty acid synthesis due to reduced synthesis of acetyl CoA carboxylase (repression) and the fatty acid synthase complex. This downregulation conserves resources when energy intake is limited.
Question 17: Explain the importance of malonyl CoA in coordinating fatty acid synthesis and beta-oxidation.
Answer: Malonyl CoA plays a crucial role in coordinating fatty acid synthesis and beta-oxidation by inhibiting carnitine palmitoyltransferase I (CPT I). This inhibition prevents the breakdown of newly synthesized fatty acids during the fed state when fatty acid synthesis is active. When malonyl CoA levels decrease, such as during starvation or in diabetes mellitus, CPT I is no longer inhibited, allowing for increased beta-oxidation.
Question 18: Where do fatty acid elongation and desaturation primarily occur within the cell?
Answer:
○ Elongation: Fatty acid elongation occurs in both the smooth endoplasmic reticulum and the mitochondria. These systems add two-carbon units to existing fatty acids, extending their chain length.
○ Desaturation: Desaturation, the introduction of double bonds into fatty acids, takes place in the smooth endoplasmic reticulum. This process requires specific enzymes, fatty acyl-CoA desaturases, along with cytochrome b5, NADH, and oxygen.
Question 19: Explain why humans require linoleic acid and linolenic acid in their diet.
Answer: Humans require linoleic acid and linolenic acid in their diet because our bodies cannot synthesize these polyunsaturated fatty acids. We lack the enzymes needed to introduce double bonds beyond the Δ9 position in fatty acids. These essential fatty acids are crucial as precursors for other important molecules, including arachidonic acid, and are involved in various physiological functions.
Question 20: Describe the sources of fatty acids used for triacylglycerol (TAG) synthesis in the liver and adipose tissue.
Answer: The sources of fatty acids used for TAG synthesis in the liver and adipose tissue include:
○ Diet: Dietary fats provide a direct source of fatty acids.
○ Adipolysis: The breakdown of stored TAGs in adipose tissue, primarily through the action of hormone-sensitive lipase, releases fatty acids.
○ De novo synthesis: Excess carbohydrates, protein, and other molecules obtained from the diet that exceed the body’s immediate needs can be converted to fatty acids and stored as TAGs.
Question 21: How does acetyl CoA carboxylase indirectly regulate beta-oxidation?
Answer: Acetyl CoA carboxylase catalyzes the formation of malonyl CoA, the first committed step in fatty acid synthesis. Malonyl CoA, in turn, acts as an inhibitor of carnitine palmitoyltransferase I, the enzyme responsible for transporting fatty acids into the mitochondria for beta-oxidation. Therefore, by controlling the production of malonyl CoA, acetyl CoA carboxylase indirectly regulates the rate of beta-oxidation.
Question 22: What would be the immediate consequence on beta-oxidation if acetyl CoA carboxylase activity were significantly decreased?
Answer: If acetyl CoA carboxylase activity is decreased, the production of malonyl CoA would also decrease. This would lead to a reduction in the inhibition of carnitine palmitoyltransferase I, allowing more fatty acids to be transported into the mitochondria for beta-oxidation. Therefore, a decrease in acetyl CoA carboxylase activity would result in an increase in the rate of beta-oxidation.
Question 23: Under what metabolic conditions might decreased acetyl CoA carboxylase activity be beneficial for an organism?
Answer: Decreased acetyl CoA carboxylase activity, leading to increased beta-oxidation, would be beneficial during periods of energy scarcity, such as fasting or prolonged exercise. In these situations, the body needs to mobilize stored energy reserves, and beta-oxidation of fatty acids is a major pathway for ATP production.
Question 24: What are the potential long-term consequences for an organism if acetyl CoA carboxylase activity remains chronically low?
Answer: While a short-term decrease in acetyl CoA carboxylase activity can be beneficial during energy scarcity, chronic low activity could lead to several issues:
Depletion of Fat Stores: Continuously high rates of beta-oxidation could eventually deplete the body’s fat stores, which are important for insulation, organ protection, and hormone production.
Metabolic Imbalances: A constant shift towards fatty acid oxidation might disrupt the balance between carbohydrate and lipid metabolism, potentially leading to altered glucose homeostasis and other metabolic disturbances.
Impaired Fatty Acid Synthesis: Although fatty acid synthesis might not be a priority during energy scarcity, it’s crucial for other processes like cell membrane synthesis and repair. Chronically low acetyl CoA carboxylase activity could impair these processes.
Question 25: How do hormonal signals like insulin and glucagon influence acetyl CoA carboxylase activity and thus impact beta-oxidation?
Answer: Hormonal signals play a crucial role in regulating acetyl CoA carboxylase activity and consequently beta-oxidation :
Insulin: In the well-fed state, high insulin levels stimulate acetyl CoA carboxylase activity, promoting fatty acid synthesis and increasing malonyl CoA levels. This leads to inhibition of beta-oxidation, favoring energy storage.
Glucagon: During fasting or exercise, glucagon levels rise. Glucagon inhibits acetyl CoA carboxylase activity, reducing malonyl CoA levels and relieving the inhibition on carnitine palmitoyltransferase I. This promotes beta-oxidation to provide energy from stored fatty acids.
Question 26: What is the function of the citrate shuttle in fatty acid synthesis?
Answer: Fatty acid synthesis requires acetyl-CoA, which is primarily produced in the mitochondria. However, the mitochondrial inner membrane is impermeable to acetyl-CoA. The citrate shuttle provides a mechanism to transport acetyl-CoA from the mitochondria to the cytosol, where fatty acid synthesis takes place1.
Question 27: What is the role of ATP citrate lyase in fatty acid synthesis?
Answer: ATP citrate lyase is a cytosolic enzyme that cleaves citrate to regenerate acetyl-CoA and oxaloacetate. This reaction is crucial because it makes acetyl-CoA available for fatty acid synthesis in the cytosol.
Question 28: What happens to the oxaloacetate produced by ATP citrate lyase?
Answer: Oxaloacetate, the other product of citrate cleavage, can have several fates:
■ It can be converted to malate, which can then be converted to pyruvate by malic enzyme. This reaction produces NADPH, a crucial reducing agent for fatty acid synthesis. Pyruvate and malate can be transported back into the mitochondria.
■ Oxaloacetate can also be used for glucose production.
Question 29: How does the regulation of the citrate shuttle and ATP citrate lyase contribute to the overall control of fatty acid synthesis?
Answer: The regulation of these processes ensures that fatty acid synthesis occurs primarily when the cell has an excess of energy and building blocks. High levels of citrate, indicating energy abundance, activate both the citrate shuttle and ATP citrate lyase, thus promoting fatty acid synthesis.
Question 30: What are the primary locations for triacylglycerol (TAG) synthesis in the body?
Answer: Liver, adipose tissues, and lactating mammary glands are the major sites of TAG synthesis.
Question 31: Besides de novo synthesis, what are the sources of fatty acids used for TAG synthesis?
Answer: The fatty acids used in TAG synthesis can come from three main sources:
- Diet: Fatty acids consumed in the diet can be directly used.
- Adipolysis: Hormone-sensitive lipase breaks down stored triglycerides in adipose tissue, releasing fatty acids.
- De novo synthesis: When the body has excess carbohydrates, protein, or other molecules, these can be converted into fatty acids and stored as TAGs.
Question 32: What is the significance of glycerol 3-phosphate in TAG synthesis, and how does its source differ between the liver and adipose tissue?
Answer: Glycerol 3-phosphate is a necessary component for TAG synthesis. In the liver, it can be synthesized from glucose through glycolysis or from glycerol through the action of glycerol kinase. However, adipose tissue lacks glycerol kinase, so the glycerol 3-phosphate used for TAG synthesis in adipose tissue must come from glucose metabolism.
Question 33: What are the key steps involved in the synthesis of TAGs?
Answer: The steps of TAG synthesis are as follows:
Step 1: Activation of fatty acids: Fatty acids are activated by converting them to acyl-CoA.
Step 2: Activation of glycerol: Glycerol is activated by converting it to glycerol 3-phosphate.
Step 3: Esterification: Two molecules of acyl-CoA are esterified to glycerol 3-phosphate, forming phosphatidic acid. A third acyl-CoA is then esterified to phosphatidic acid to yield TAG.
Question 34: How is the fate of synthesized TAGs different in the liver compared to adipose tissues?
Answer: While both the liver and adipose tissues synthesize TAGs, their purposes differ:
Liver: TAGs synthesized in the liver are primarily packaged into very low-density lipoproteins (VLDL) and transported to other tissues for utilization or storage.
Adipose tissues: TAGs synthesized in adipose tissues are primarily stored within the adipocytes for later use as an energy source.
Question 35: How does the interplay between the regulation of fatty acid synthesis and beta-oxidation ensure efficient energy utilization in the body?
Answer: The regulation of fatty acid synthesis and beta-oxidation is coordinated to prevent futile cycling. Malonyl-CoA, a key intermediate in fatty acid synthesis, inhibits carnitine palmitoyltransferase I, the enzyme responsible for transporting fatty acids into the mitochondria for beta-oxidation. In a well-fed state, high levels of malonyl-CoA promote fatty acid synthesis while inhibiting beta-oxidation. During starvation or when energy demands are high, malonyl-CoA levels decrease, relieving the inhibition of beta-oxidation and allowing fatty acid breakdown to provide energy.
Question 36: Why is the transportation of acetyl-CoA from the mitochondria to the cytosol a crucial step in fatty acid synthesis? What are the implications of this transport system for cellular metabolism beyond fatty acid synthesis?
Answer: Acetyl-CoA, the precursor for fatty acid synthesis, is primarily produced in the mitochondria through the breakdown of carbohydrates and fatty acids. However, fatty acid synthesis occurs in the cytosol. The citrate shuttle facilitates the transport of acetyl-CoA to the cytosol by converting it to citrate, which can cross the mitochondrial membrane. Once in the cytosol, citrate is cleaved back to acetyl-CoA by ATP citrate lyase. This transport system not only provides the building blocks for fatty acid synthesis but also links carbohydrate metabolism to lipid metabolism. The oxaloacetate produced from citrate cleavage can be used for gluconeogenesis or converted to malate, generating NADPH for fatty acid synthesis.
Question 37: Explain the rationale for the multiple levels of regulation of acetyl-CoA carboxylase, the rate-limiting enzyme in fatty acid synthesis.
Answer: Acetyl-CoA carboxylase is a key control point in fatty acid synthesis, subject to allosteric regulation, feedback inhibition, and hormonal control through phosphorylation. This multi-level regulation ensures that fatty acid synthesis is tightly controlled and responsive to cellular energy status and hormonal signals. Citrate, an indicator of abundant acetyl-CoA, allosterically activates the enzyme, while palmitoyl-CoA, the end-product of fatty acid synthesis, provides feedback inhibition. Hormonal regulation by insulin and glucagon further fine-tunes the activity of the enzyme in response to feeding and fasting states.
Question 38: How does the body synthesize longer-chain and unsaturated fatty acids from palmitate, the primary product of fatty acid synthase? What is the significance of these modifications in terms of fatty acid diversity and function?
Answer: Palmitate, a 16-carbon saturated fatty acid, serves as the precursor for the synthesis of longer-chain and unsaturated fatty acids. Elongation occurs in the endoplasmic reticulum and mitochondria, adding two-carbon units using malonyl-CoA as a donor. Desaturation, the introduction of double bonds, takes place in the endoplasmic reticulum and requires molecular oxygen and NADH. These modifications increase the diversity of fatty acids, contributing to the structural and functional complexity of lipids. Unsaturated fatty acids, for instance, play a crucial role in membrane fluidity and are precursors for signaling molecules.
Question 39: Compare and contrast TAG synthesis in the liver and adipose tissues, considering the sources of fatty acids, the role of glycerol 3-phosphate, and the fate of synthesized TAGs.
Answer: Both liver and adipose tissues synthesize TAGs, but with distinct roles. The liver uses fatty acids from the diet, de novo synthesis, and, to a lesser extent, lipolysis. Adipose tissue relies on dietary fatty acids and lipolysis. Glycerol 3-phosphate, essential for TAG synthesis, can be produced from both glucose and glycerol in the liver. Adipose tissue, lacking glycerol kinase, relies solely on glucose-derived glycerol 3-phosphate. The liver packages synthesized TAGs into VLDL for transport to other tissues, while adipose tissue stores TAGs as an energy reserve. This division of labor highlights the liver’s role in lipid distribution and the adipose tissue’s function in energy storage.
